Purpose
Standard therapies for grade 3 IDH mutant, 1p/19q co-deleted oligodendrogliomas involve maximum safe surgical resection (MSR), followed by adjuvant, standard fractionation external beam radiation therapy (EBRT) from 59.4 to 60 Gy with chemotherapy. Currently, procarbazine, lomustine, and vincristine (PCV) are favored, having demonstrated survival benefit to EBRT, as shown by RTOG 9402 [1, 2] and EORTC 26951 [1, 3]. However, current research focuses on considering temozolomide as a more well-tolerated alternative (CODEL trial), with a large retrospective analysis showing equivalency [4]
There has been renewed interest in intra-cranial brachytherapy with the development of GammaTile® (GT Medical Technologies, Tempe, Arizona, USA). This permanent implantation platform differs from older strategies by suspending evenly spaced cesium-131 (131Cs) radioactive seeds in a collagen-based sponge vehicle [5, 6]. Incorporating a barrier between radioisotope seeds and the brain parenchyma reduces potential hot spots, thus reducing radionecrosis risk, an issue with prior implanted brachytherapy techniques. The ease of evenly distributing tiles with pre-determined seed spacing also improves overall coverage and dose homogeneity. Emerging data demonstrate the efficacy and safety of GammaTile in re-irradiation in patients with grade 3 gliomas and glioblastoma [7, 8]. As such, GammaTile delivers more dose to residual cancer cells near the resection bed, which has shown potential enhanced survival in salvage glioblastoma treatment [8] compared with external beam [9] and re-resection [10]
Controversy exists regarding the implementation of radiation therapy in patients with connective tissue disorders (CTDs), such as scleroderma. CTDs, also known as collagen vascular disorders, are relative or absolute contraindications for radiation therapy [11, 12] due to potential greater risk of radiotherapy-induced dermatologic complications [13]. As the pathophysiology of scleroderma decreases from vascular fibrosis and enhanced inflammation at baseline, the theoretical prospective for radiotherapy to exacerbate this underlying pathology has been proposed as a probable mechanism [13]
In this case report, we present the first application of GammaTile® brachytherapy to an intra-cranial tumor in a patient with limited scleroderma. We demonstrate significant dose reduction to the surrounding scalp, surgical scar, and normal brain parenchyma, compared with a standard EBRT plan and similar reduced volume EBRT plan. Finally, we illustrate the lack of clinical and radiographic toxicities on follow-up
Case description
A 49-year-old African American woman presented with focal epilepsy with loss of consciousness. Her past medical history was significant for limited scleroderma, with diffuse skin tightening, contractures of the hands, skin hypopigmentation including the scalp, alopecia, and multiple system involvement with hypertension, inflammatory arthritis, Raynaud’s syndrome, gastrointestinal dysmotility, and polymyositis. The patient was maintained on tofacitinib and intravenous immunoglobulin (IVIG) due to disease severity. A brain MRI upon workup demonstrated a 3.8 × 5.2 × 3.7 cm ring-enhancing, FLAIR hyperintense mass with cystic component in the right frontal lobe (Fig. 1A, B). Stereoscopic biopsy demonstrated an IDH-mutated, 1p/19q co-deleted grade 3 oligodendroglioma with MGMT promoter hypermethylation. There was a concern that EBRT would lead to impaired wound healing from her craniotomy and excessive dermal toxicity to the scalp overlying the irradiated surgical bed, given her severe scleroderma. Since small vessel damage is an integral part of brain radionecrosis, there was also a concern regarding an increased risk of radionecrosis when applying traditional EBRT volumes that include a 1-1.5 cm ring of normal brain parenchyma. Multidisciplinary central nervous system (CNS) tumor board consensus was to proceed with GammaTile to reduce dose to the incision, scalp, and normal brain tissue. The patient subsequently underwent MSR with placement of 10 tiles containing 40 131Cs seeds (average, 5.47 mCi per seed) around the periphery of the surgical cavity to deliver a prescribed 60 Gy to a 5 mm depth (Figure 2). As demonstrated in the overlay of the day 1 post-operative CT scan and T1 post-contrast MRI, the dense seeds highlighted by the CT portion evenly covered the surgical cavity, with no significant residual disease (Figure 1C).
Fig. 1
Pre-operative and post-operative imaging of grade 3 glioma. Axial pre-operative MRI demonstrate peripheral enhancement on T1 post-contrast MRI (A) with signal throughout the tumor of T2-FLAIR MRI. B) Post-operative day 1 CT (C) is over- laid on top of the post-operative day 1 T1 post-contrast MRI, showing little enhancement around the resection cavity with even distribution of GammaTile seeds along surgical margin (high CT signal highlighted by the green arrow)
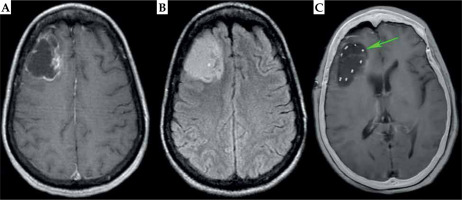
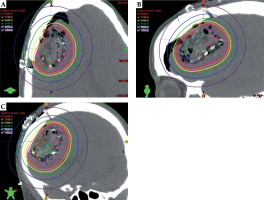
Fig. 2
Seed arrangement and post-implant dosimetry of treated grade 3 glioma in the axial (A), sagittal (B), and coronal (C) axis. The isodose lines show rapid dose fall- off past the 60 Gy prescription volume. The individual tile arrangement is demonstrated by outlining the seeds from each tile with the same color
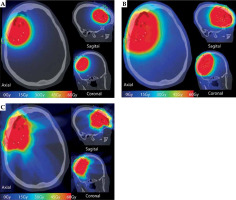
The GammaTile dose cloud was converted to equivalent dose of 2 Gy (EQD2), as shown in Figure 3A, with prescription dose delivered conformally around the surgical bed, sharp dose fall-off into the surrounding brain parenchyma, and dose shielding to the scalp from the overlying calvaria. For comparison, we generated a standard EBRT plan consisting of an overall 1.5 cm expansion to 59.4 Gy in 1.8 Gy fractions (Figure 3B). A traditional EBRT plan resulted in significantly slower dose fall-off compared with GammaTile, with visually greater dose to the scalp. We also developed a CyberKnife (Accuray) plan with a 5 mm expansion on the surgical cavity prescribed to 60 Gy in 30 fractions, to make an EBRT comparison using the same prescription volume as GammaTile. CyberKnife, by virtue of its non-coplanar delivery, allows for sharper dose fall-off than traditional linear accelerators. Nevertheless, the CyberKnife volume resulted in irradiation of a greater amount of brain tissue, with significant entry dose into the scalp (Figure 3C).
Fig. 3
Dose cloud received by the patient with GammaTile (A) as well as the hypothetical dose cloud from a traditional EBRT volume (B) and EBRT volume delivered with CyberKnife (C) to the same 60 Gy GammaTile prescription volume. Compared with EBRT dose clouds, there is visually less dose to the scalp, incision scar (green line), and brain parenchyma with GammaTile delivery
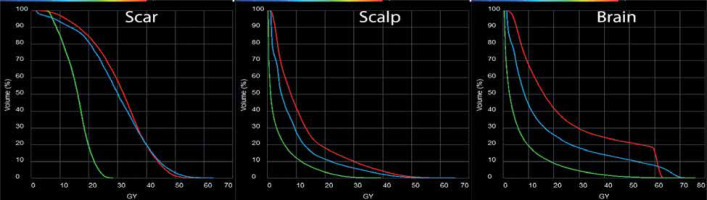
To obtain a quantifiable dose comparison, organs at risk (OARs), including the scalp, scar, optic structures, and other CNS structures were contoured on the delivered GammaTile plan and hypothetical plans. The resulting dose volume histograms indicated that standard volume EBRT delivered a significantly greater amount of high/moderate-dose to the resection scar and scalp, as compared with GammaTile (Figure 4A, B). The CyberKnife volume was able to reduce the overall scalp dose compared with the standard EBRT; however, there was not a large difference in the resection scar dose. Figure 4C demonstrates significant reduction of dose to normal brain tissue with GammaTile compared with standard volume EBRT. The CyberKnife volume delivered an intermediate-dose at the cost of a greater hot spot between 60-70 Gy to the normal brain tissue. In general, the maximum dose delivered to the scar and scalp by GammaTile was reduced to half, when compared with both external beam techniques of approximately 25-30 Gy vs. 55-60 Gy. The relative amount of dose to other OARs followed the same overall trend, except for the right cochlea, where the maximum dose from GammaTile was significantly lower at 3.0 Gy vs. 11.9 Gy for EBRT and 15.1 Gy for CyberKnife.
Fig. 4
Dose-volume histogram (DVH) comparison between GammaTile delivery (green), standard volume EBRT (red), and reduced volume EBRT (blue) to the scar (A), scalp (B), and brain parenchyma (C). In all cases, the utilization of GammaTile significantly reduced the dose to these structures
Given the patient comorbidities from scleroderma and reduced performance status, the patient opted for single-agent temozolomide therapy in 12 cycles. At 1-, 3-, 6-, and 12-month post-surgery, there was no acute dermatologic toxicity observed, with no impairment in incisional healing (Figure 5A, B). The patient did not have any seizures post-operatively, and she did not develop new or worsening neurologic deficits. MRI brain at 6 months (Figure 6A,B) demonstrated stable post-treatment effect around the surgical cavity, without evidence of new or progressive disease, and without evidence of radionecrosis or soft tissue edema. MRI brain at 12 months (Figure 7A, B) showed stable enhancement along the surgical cavity. The mildly expansile, confluent FLAIR hyperintensity surrounding the resection cavity slowly increased over time, without significant mass effect. These findings are more consistent with evolving post-treatment changes rather than tumor progression.
Fig. 5
Photograph of the surgical incision and radiotherapy field at 6 months (A) and 12 months (B) post-operatively. The green circle approximates the area of scalp above the irradiated region that received the prescription dose to 60 Gy. The red arrows signify the surgical incision. There are no skin changes to the scalp from baseline, and the scar is well-healed with no excessive scarring
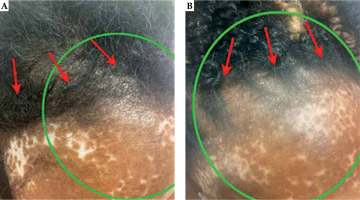
Fig. 6
Six months post-operative MRI demonstrates stable linear enhancement around the surgical cavity in the right frontal lobe related to post-surgical changes on T1 post-contrast MRI (A). Similarly, T2-FLAIR (B) shows minimal surrounding edema without mass effect. Both sequences do not demonstrate evidence of radiation necrosis
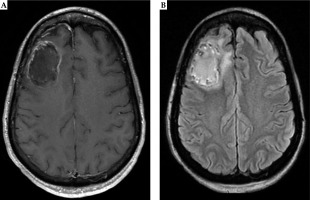
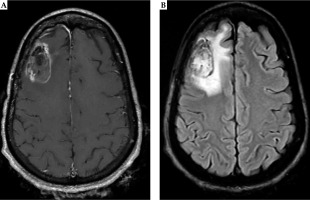
Fig. 7
Twelve months post-operative MRI shows stable enhancement around the surgical cavity on T1 post-contrast MRI (A). There is a slight increase in the surrounding FLAIR hyperintense signal without mass effect. Mildly expansile, confluent FLAIR hyperintensity surrounding the resection cavity is increased, and may represent evolving post-treatment change (B)
Discussion
While EBRT is a relative contraindication for patients with scleroderma and a relative to absolute contraindication in other CTDs, brachytherapy with 131Cs (GammaTile) is a CNS radiotherapy option in this patient population. Already, this platform has been documented in multi-institutional reports to offer very low rates of radionecrosis and wound complications for various intra-cranial lesions [14]. In the present work, we presented the first reported case of GammaTile utilization for intra-cranial radiation in an oncology patient with severe, symptomatic scleroderma. Skin irritation is reported as one of the most common side effects of GammaTile [14]; however, we showed that the dose to the scalp of the patient was very low, which was partially due to the overlaying, high density calvaria acting as a shield. In contrast, EBRT, in requiring entry dose through the scalp and slower dose fall-off from PTV, results in significant dose escalation to the scalp and resection scar, even when using similar treatment volumes to GammaTile.
GammaTile delivers almost negligible dose to the adjacent cochlea compared with EBRT, which may be clinically relevant given that hearing loss is a possible manifestation of the scarring caused by scleroderma [15]. The low-dose GammaTile delivered to the scalp and scar did not result in acute skin changes or wound healing impairment at the overlaying incision at 3-, 6-, and 12-month follow-up. GammaTile also dramatically decreased the dose to surrounding brain parenchyma, when compared with EBRT plans, possibly contributing to the lack of appreciable radionecrosis radiographically through 12 months, as observed in other reports [16]. These findings also strengthen the dosimetric advantages of 131Cs over previously utilized iodine-125 (125I) [17-19], leading to a decrease in observed radionecrosis with 131Cs and 125I, 8.4% radiographic [20] vs. 23% symptomatic, respectively [21].
Scleroderma and other CTDs are at increased risk of both acute and chronic toxicities with radiotherapy [11]. Therefore, future publications will focus on long-term follow-up of this patient to determine chronic side-effects in addition to disease control. To further characterize both the safety and efficacy in patients with CTD, future efforts will also be made to create a prospective cohort of such patients treated at multiple GammaTile centers showing a multi-institutional perspective. Current published reports on re-irradiation of gliomas have suggested that the efficacy and safety will persist in patients with improved survival [8] compared with other surgical [10] and salvage EBRT [9] in an already irradiated population.
Collectively, we have demonstrated the short-term safety and set a precedent of GammaTile brachytherapy for CNS radiation in patients with scleroderma. This is a brachytherapy strategy with valuable pragmatic clinical implications that should be considered when treating intra-cranial neoplasms in patients with CTDs.


