Introduction
Basal cell carcinoma (BCC) is the most common skin cancer, especially fair-skinned adults, and the lifetime risk of its incidence in this population is estimated to be approximately 30% [1]. Because BCC mainly affects older people and as the aging population of the world increases with longer life expectancies, this cancer will continue to affect more and more people [2, 3]. The worldwide frequency of BCC cannot be precisely evaluated because this neoplasm is not consistently registered [1, 3, 4].
Basal cell carcinoma is considered a slow growing tumour; however, its enlargement is observed as time passes [5, 6]. Although BCC is highly treatable and the risk of mortality is low, the level of significant morbidity is high due to local tissue destruction and invasion [3, 6]. The severity of this disease is also related to its localization; on the scalp, it can grow for years without being noticed due to the hairy skin, and the periocular area is recognized as a high-risk site [3, 5]. Treatment of large tumours is generally associated with the complexity of surgical reconstruction and is a financial burden on the health care system [3, 6].
Basal cell carcinomas are a heterogeneous group of neoplasms, with different histopathological characteristics that range from superficial lesions to very aggressive tumours [1]. Basic categorization divides BCC into 2 main types of cancer: 1) non-aggressive BCC, superficial and nodular histopathologic types, and 2) aggressive BCC, infiltrative, micronodular, metatypical, and mixed histopathologic types [1, 5]. It is suspected that the BCC subtypes have different biological behaviour [7]. One of the most significant differentiating factors of the biological pathways of the BCC subtypes is the cancer growth rate. Methods to measure tumour growth speed over time can be based on patient interviews or measurements of neoplasm dimensions during the first visit and then at the time of the surgical procedure [4, 5, 7–9].
Knowing the growth rate of BCC, especially in relation to BCC subtypes, seems to be highly relevant. Patients with skin cancers are generally older, have other comorbidities, and often they refuse to undergo medical treatment. Recognizing the speed of growth of particular BCC subtypes may allow us to guide patient treatment in a reasonable manner with an acceptable risk of cancer growth.
We present a meta-analysis and systematic review summarizing the size of the growth rate in BCC, depending also on its subtypes.
Material and methods
Search strategy
To find all relevant studies, online medical databases such as PubMed, Scopus, Embase, Web of Science, and Google Scholar were searched regarding the growth rate of BCC. In agreement with the Boolean technique, the following search terms were employed: (basal cell carcinoma growth rate) or (basal cell carcinoma growth) or (basal cell carcinoma proliferation rate) or (basal cell carcinoma growth factor). No dates, language, article type, or text availability conditions were applied. To ensure the precision of the search, an additional search was carried out through the references of the identified studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed during the study.
Eligibility assessment
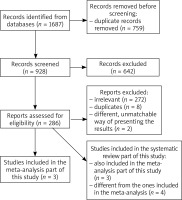
To establish the growth rates of BCC, a total of 1677 studies were first reviewed by 2 independent reviewers, including a search of the essential database and an additional manual search through the references. A total of 286 articles were eligible for full text examination. To minimize potential bias and maintain accurate statistical methodology, the exclusion criteria involved articles such as case reports, case series, conference reports, reviews, letters to the editors, and studies that provided incomplete or irrelevant data. The inclusion criteria involved original studies with complete and extractable data considering BCC growth rates. The data collection process is shown in Figure 1.
Data extraction
Three independent reviewers extracted data from qualified studies. Qualitative data were collected, such as year of publication, country, continent, and descriptions of the BCC growth rates. Quantitative data, such as the growth rate of the long axis, the growth rate of the short axis, and the area growth rate of the BCC, were gathered. Two studies with quantitative data were excluded from the meta-analysis part of this study because their way of presenting the data differed from the other results and, therefore, it was impossible to compare them appropriately statistically. The authors of those publications were contacted, but raw data on growth rates were not available. Studies that contained mean results without standard deviation or interquartile range or unclear or unspecified growth rates were excluded. Any discrepancies between the studies identified by 2 independent reviewers were resolved by contacting the authors of the original studies whenever possible or by consensus with a third reviewer.
Statistical analysis
To perform statistical analysis, Comprehensive Meta-analysis version 3.0 software (Biostat Inc., Englewood, NJ, USA) was used. A random-effects model was performed in all analyses. The χ2 test and I2 statistics were used to assess the heterogeneity among the studies. A p-value was used to determine statistical significance between studies. A p-value less than 0.05 was considered statistically significant.
Results
A total of 7 studies were included in this review. Five studies contained data on the growth rate of basal cell carcinomas. Unfortunately, due to differences in the way of presenting the results, it was impossible to compare 2 of those studies with others. Therefore, these studies were excluded from the meta-analysis part of this study. However, both articles were encountered in the systematic review part of this article. After eliminating those 2 studies, there were too few data on the growth rate of the short axis and the growth rate of the BCC area. Therefore, those analyses were not performed.
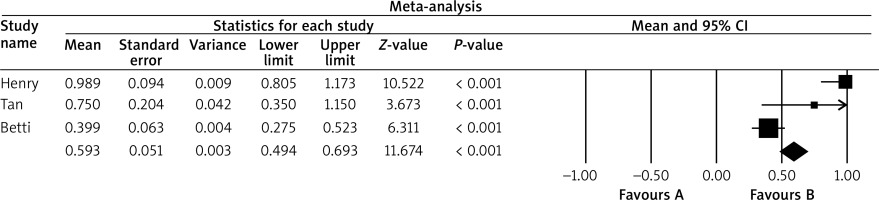
The results of the remaining 3 studies were deeply analysed [5, 7, 8]. The characteristics, summary, and some data extracted from all studies can be found in Table 1. The descriptive information from all studies was thoroughly analysed and presented in the discussion [4–10]. The mean growth rate of the BCCs was established at 0.71 mm/month (standard error: 0.22). Detailed results are shown in Table 2. The results were also displayed on a forest plot, showing the distribution of growth rates of both the submitted studies and our results (Figure 2).
Table 1
Characteristics of submitted studies. Materials required to fill this table come from the submitted articles [4–10]
Discussion
The aim of this paper was to meta-analyse and systematically review the growth rate of BCC depending also on its subtypes. In the meta-analysis, 3 articles were taken into account [5, 7, 8].
Teloh was one of the first to take interest in the issue of the growth ratio of BCC [8]. The author measured the growth ratio according to the equation in which the numerator was the size of the tumour in millimetres and the denominator was the duration of the tumour in months. He gathered a group of 100 patients with BCC: 71 men and 29 women. In both sexes, the growth rate was 1.12 mm/month. He did not calculate the growth ratio in different histopathological subtypes of BCC; however, he compared it to different histological characteristics. Rapidly growing neoplasms were characterized by noticeable inflammation and invasion of surrounding tissues, whereas slowly growing BCC presented a peripheral palisading, cystic, and adenoid change [8].
Betti et al. presented a group of 44 patients with different types of BCC [7]. The authors measured the maximum lateral and depth extension of surgically removed cancers in millimetres based on histological samples, and defined the growth rate as the ratio between the maximum lateral extension and/or depth in millimetres of the tumour and the tumour growth time in months. Their results indicated that there is a significant difference between the infiltrative/micronodular and nodular subtypes of BCC versus the superficial subtype of BCC with respect to depth extension, and a significant difference between the nodular subtype of BCC versus the superficial subtype of BCC with respect to lateral extension. This is a confirmation of the typical clinical picture. Furthermore, they noticed that the growth rate for depth and lateral extension was highest for the nodular type of BCC (0.278 mm/month), while for the superficial type of BCC the depth extension was the smallest (0.075 mm/month), and for the infiltrative type of BCC the lateral extension was the smallest (0.377 mm/month) [7]. The authors paid attention to the fact that local conditions can favour a certain subtype of BCC, restricting the depth of superficial BCC and accelerating the growth of nodular and infiltrative BCC [7].
Because the duration of the tumour in months is sometimes difficult to establish, it is worth finding a highly objective and reliable method of measuring the speed of cancer growth. Tan et al. measured tumour dimensions at the first specialist appointment and on the day of Mohs surgery. Then, based on these 2 measurements, the growth ratio was calculated [5]. Furthermore, at the first visit, all patients had a shave or incisional biopsy of the tumour, so the histological type of BCC was also recorded. The authors based their work on the group of 115 patients with periocular BCC. The average waiting time for Mohs surgery was 157 ±87 days, and the growth rate of BCC was 0.76 mm in diameter per month. Non-aggressive BCCs were defined as nodular or superficial type, and aggressive BCCs were defined as infiltrative, micronodular, metatypical, or mixed subtypes. The authors revealed that factors related to faster growth were large initial cancer size, male sex, and recurrent tumours [8]. They concluded that the periocular area is considered as a high-risk side of BCC, and at this location it grows rapidly, and many BCCs here have aggressive histological subtypes [8].
Sykes et al. performed a retrospective review of clinical and dermoscopic images taken in patients attending a skin lesion diagnostic service [4]. The authors collected data for 100 superficial BCC in 70 patients. The median surface area was 41.9 mm2 with a growth rate of 0.81 mm2/month. Males had larger BCC dimensions than females [4]. The presented study supported the opinion that BCC is a slow-growing cancer, especially its superficial subtype, at a rate of less than 1 mm2 per month, and it was based on an objective method such as dermoscopy.
Kricker et al. [9] analysed the largest group of patients with BCC. The authors presented data for 887 patients with BCC. The size of each lesion was determined only at the time of excision and recorded by the reporting pathologist. Time was measured from the date of first notice of the lesion by the patient or physician. The authors found that BCCs increased in size with a longer time between first noticing and excision. Other independent correlates of larger BCCs when excised included older age, male sex, having no skin checks by the doctor, having a morphoeic or micronodular tumour, ulceration, and scar tissue with injury. The authors noticed that BCC increased progressively in size over time and was more likely to be smaller when patients had 4 to 6 monthly skin checks by a physician, suggesting that early diagnosis of BCC can lead to smaller tumours and possibly less extensive treatment and better outcomes. The direct association of scar tissue with larger BCCs shows the importance of complete excision in the first attempt [9].
Lee et al. [6] showed a slightly smaller group of patients. The authors presented 802 cases of BCC treated with Mohs surgery: 228 of them aggressive and 540 indolent in histopathology. The median change in the main diameter for all lesions was 0.8 cm (mean 0.9 cm). Furthermore, the independent predictors of the major change in diameter included age, gender, aggressive histology, and previous treatment [6].
Stamp et al. presented data on the role of TGF-β in patients with BCC [10]. The analysis revealed that TGF-β was present in the stroma surrounding the BCC in most cases (29 out of 50) [10]. The authors concluded that many of the characteristics of BCC, mainly its growth, may be explained by the effects of TGF-β.
The main limitation of the presented data is the heterogeneity among the included studies, which allowed us to include only 3 articles in the meta-analysis, and the rest were systematically reviewed. One of the reasons for the heterogeneity is the different methodologies used by the authors in their research. Some of the authors calculated the growth rate according to mathematical equations, while others used tools like dermoscopy. The number of patients examined in the analysed groups also varied and only in some was there a division depending on the BCC subtype.
In conclusion, the presented analysis shows that BCC is generally a slow-growing tumour, with a mean growth rate of about 0.7 mm/month. However, it was proven that this growth rate is different depending on the BCC subtype. Neoplasms recognized as aggressive subtypes, such as infiltrative, micronodular, metatypical, or mixed, tend to have a higher growth rate than nonaggressive BCC, such as the nodular or superficial type. Additionally, there are some high-risk areas in which BCC can be localized and grow quite rapidly; this is mainly the periocular area and the face in general. In addition, it should be remembered that tumours in males have larger dimensions than in females, which was also observed by Fijałkowska et al. [3]. From the results obtained, it might be suggested that on a first consultation, when a patient has a tumour suspected of BCC, it is worth taking a biopsy to verify one’s suspicion and check the subtype of BCC. Then, depending on the result and location of the tumour, a faster or slower decision of excision can be made. Further studies that present a comparable methodology are needed to strengthen the conclusion in a larger group of patients.