Purpose
Locally recurrent breast cancer after conservative surgery and adjuvant whole-breast external beam radiotherapy (EBRT) is an uncommon event, which historically has been reported in around 6% in node-negative patients (or less, as in the control arm of ELIOT – intraoperative radiotherapy with electrons trial) and in 10% in node-positive patients at 5 years, or even lower in more recent trials [1,2]. Studies of the patterns of failure after conservative approach demonstrated that the same quadrant as the primary tumor is most frequently affected by an ipsilateral breast tumor recurrence (IBTR). Whatever it is seen as a true relapse or as a new tumor, the standard of care is mastectomy, with or without post-operative reirradiation, which ensure a local control of 68-98%. Alternative approaches are under investigation [3,4,5,6,7,8,9]. According to a recent review, after a second breast conserving surgery (SBCS) alone, the weighted rates for 5-year local control, distant metastasis-free survival, and overall survival were 76%, 73%, and 77%, respectively [10].
At our institute, after a SBCS approach, recurrent tumor size ≤ 2 cm, Ki-67 < 20%, and time to relapse > 48 months were associated with an increased disease-free survival, whereas absence of estrogen receptors affected overall survival [11,12].
The addition of a further course of adjuvant radiotherapy after SBCS might reduce the risk of local recurrence, and some recent guidelines have strongly suggested to consider the conservative approach including reirradiation along with salvage mastectomy. The possibility of an early diagnosis and the availability of high precision EBRT techniques offered by current technological advances has paved the way for salvage breast conservation based on wide local excision partial breast reirradiation [13,14,15,16,17,18,19,20,21,22,23,24,25].
The conservative approach can be proposed after careful evaluation of surgical feasibility, which must consider the IBTR dimension, size, focality, and the breast size in order to achieve cosmetically acceptable results. Several authors have described the use of adjuvant breast reirradiation with brachytherapy (BT) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], intraoperative radiation therapy (IORT) [41,42,43,44,45,46], proton beam radiotherapy [47,48], or EBRT with or without concurrent hyperthermia to increase local control rate [49,50,51,52,53,54].
Brachytherapy can be delivered using low-dose-rate (LDR), pulsed-dose-rate (PDR), or high-dose-rate (HDR) modality. While with LDR technique irradiation is delivered continuously, with HDR and PDR BT the dose is given with a fractionation schedule using afterloading equipment [55].
The purpose of this study, called BALESTRA (Brachytherapy as Adjuvant Local rEirradiation for Salvage Treatment of Recurrent breAst cancer), was to report on acute and late toxicity and preliminary outcome of a single-institution experience of accelerated partial breast reirradiation with adjuvant interstitial HDR-BT.
Material and methods
Patient, tumor, and treatment characteristics
The inclusion criteria were as follow: 1. Patients treated with a SBCS for ipsilateral unicentric in-breast-recurrence after conservative surgery and adjuvant whole-breast EBRT; 2. Multidisciplinary tumor board to confirm the treatment strategy; 3. Written informed consent. In addition, patients gave consent for the use of their anonymized data for research and educational purposes. Patients’ evaluation included complete medical history and physical examination. For each patient, tumor (primary and recurrence) and treatment characteristics, such as histology, recurrence site, TNM classification [56], time interval between primary tumor and relapse, doses of EBRT, and BT implant features were collected.
All the procedures were carried out using afterloading technique under local or general anesthesia. Stainless steel guide needles and/or vascular catheters were implanted through the skin, parallel to the chest wall and to each other, under ultrasound and manual control. Subsequently, polyethylene afterloading catheters were inserted through each needle into the tissue followed by removal of the needles themselves. All the catheters were secured using plastic buttons without skin sutures. Once the implantation was completed, a computed tomography (CT) scan with 2.5 mm slice thickness and separation was performed and entered into BT treatment planning system (Oncentra Brachy Planning, Nucletron-Elekta) allowing reconstruction of the catheters, definition of the target volumes and of the nearby organs at risk. No patients received intravenous contrast. The clinical target volume (CTV) included nearly 1-2 cm of healthy breast tissue around the tumor bed in all directions, with a distance between the implant and thoracic wall and/or skin of at least 5-7 mm [57,58,59,60]. Computerized optimization of dwell positions and times of stepping source was performed for fine tuning of isodose distributions. Treatment was delivered using an HDR afterloader (MicroSelectron, Nucletron-Elekta), containing a single Iridum-192 (192Ir) source. Before every BT session, the implant was examined to ensure that there was no critical displacement of the catheters. A total dose of 34 Gy in 10 fractions, 2 fractions per day, with a minimum interval of 6 hours in-between was administrated. At the end of the last fraction, the implant catheters were removed.
Endpoint analysis
All patients were followed up every three to six months to analyze acute and late toxicity, according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) scoring system [61], local control, and survival. The median follow-up was calculated from the end of BT to the last clinical observation. Local control (LC), which was defined as the absence of disease in the breast after conservative salvage surgery and BT. Progression-free survival (PFS) was calculated from the salvage surgery to the time of local, nodal and/or distant relapse, or death due to breast cancer, whichever occurred first. Cancer specific survival (CSS) and overall survival (OS) were calculated from the salvage surgery to the time of death due to breast cancer and death due to any cause, respectively.
Cosmetic results were reported using the National Surgical Adjuvant Breast and Bowel Project (NSABP)/RTOG breast cosmesis grading scale: excellent, good, fair, and poor [62].
Results
Between January 2011 and September 2015, 31 consecutive patients with histologically confirmed IBTR were treated with adjuvant interstitial HDR-BT. Patients, tumor, and treatments characteristics are listed in Table 1. Median age at first diagnosis was 46 years (range, 36.1-61.4 years). Initial surgical treatment was quadrantectomy and sentinel node biopsy with or without axillary dissection, and the median time to SBCS was 11.9 years (range, 2.5-27.8 years). The median dose of previous radiation therapy was 60 Gy (range, 50-66 Gy).
Table 1
Patients and tumor characteristics
Ipsilateral breast tumor recurrence occurred at or near the same quadrant in 22 (71%) patients, while in 9 (29%) patients was detected in a different quadrant of the breast. Primary tumor and locally IBTR showed the same histological type in 18 (58%) patients, while this information remained unknown for 2 patients (6.4%).
Histological grade 3 was found in 10 (32%) and 6 (20%) patients with primary tumor and IBTR, respectively. A second surgical axillary staging was performed in only 12 patients (39%).
None of the BT implant was performed during a surgical procedure; the median interval between salvage surgery and BT was 3.6 months (range, 1-8.2 months).
The median number of catheters and planes was 9 (range, 6-25 catheters) and 2 (range, 1-4 planes), respectively. Dosimetric records were accessible for all, except for one patient. No technical problems, such as collapses or kinking of flexible catheters allowing temporary or definitive breakdowns of treatment were recorded. Radioprotection of staff was completed for each application. No complications involving the implant, such as bleeding or infection were noted. The procedure was well tolerated in all patients. None of the patients experienced severe pain or discomfort during treatment, and no BT-related infection was observed.
Local control and survival rates
No patients were lost to follow-up. After a median follow-up of 73.7 months (range, 28.8-102.4 months), OS and CSS were 87.1% and 90.3%, respectively; 5-year LC and 5-year PFS were 90.3% and 83.9%, respectively.
Further IBTR with or without concomitant regional and/or distant metastases occurred in 3 patients (9.7%) after 32, 39, and 44 months from SBCS: two of them were successfully salvaged with mastectomy, while a patient with concomitant distant metastasis received chemotherapy only. Two patients developed distant metastases.
All but one of patients with a second relapse and/or metastasis had a Ki-67 ≥ 20% at first IBTR (4 out of 20 patients, 20%). Three of them had a histological G3 primary tumor (3 out of 10 patients, 30%) and developed the IBTR in a quadrant other than the initial primary cancer (3 out of 9 patients, 33%).
Due to small number of patients, we did not find any other association between second relapse and tumor stage, histologic type, hormonal receptor status, or dosimetric parameters.
Morbidity and cosmetic results
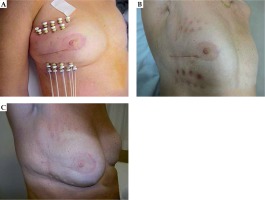
No acute skin or soft tissue side effects higher than grade 2 were recorded, all were treated topically. All patients presented good cosmetic results (Figure 1).
Probably, their unexpectedly positive judgment was intrinsically and psychologically influenced by the conservative result (even with a second surgery and a reirradiation) compared to the one they would have had if they had had to undergo a mastectomy.
Most patients showed mild late side effects (grade ≤ 2), cutaneous and sub-cutaneous fibrosis (77.4%), retraction (24%), and telangiectasia (13.7%); only one patient developed grade 3 breast edema (Table 2). Asymptomatic fat necrosis was detected in one patient and required no surgical intervention. As stated by Wazer and colleagues, skin and subcutaneous toxicities (including fibrosis) are usually associated with the V150 and V200, and inversely associated with the dose homogeneity index (DHI): DHI = (V100 – V150)/V100, where V100, V150, and V200 are CTV volumes receiving 100%, 150%, and 200% of the prescribed dose, respectively [63].
Table 2
Late toxicities (≥ 6 months after BT) observed during follow-up (n = 31 patients)
In the group of patients with grade 2 fibrosis and in the group with G0-G1 fibrosis, the median V150, V200, and DHI were 17.1 cm3 and 16.5 cm3, 7.5 cm3 and 7.6 cm3, and 0.27 and 0.4, respectively. The patient with fat necrosis received a large BT implant with 18 catheters in 3 planes; her V150 and V200 were 59.9 cm3 and 30.2 cm3, respectively (Table 3). Late breast or chest wall pain of grade 2-3 were reported in 4 patients. No pleuritis, pneumonitis, pericarditis, or rib fracture were observed.
Discussion
Local failure after breast conserving surgery and radiotherapy represents a challenge for surgeons and radiation oncologists to minimize morbidity while maintaining optimal treatment outcomes. The importance of quality of life and body image require a mandatory attention given to conservative surgical treatment of local recurrence.
Compared to EBRT, the main advantages of BT, with the radiation sources directed on the tumor bed, include a higher localized dose around the target volume and a shorter overall treatment time. The rapid fall-off of doses around sources allows relative sparing of critical normal tissues. The main disadvantage of the partial breast reirradiation is the potential not-treatment of cancer occult foci in areas of the breast outside the implanted volume. Our analysis showed that adjuvant HDR-BT is a feasible treatment for recurrent breast cancer, offering low complications rate and good cosmesis. At last follow-up, the OS and PFS were 87.1% and 83.9%, respectively, with LC of 90.3%.
We have observed an increased risk of second relapse or metastasis in patients with a histological G3 primary tumor and/or an aggressive IBTR (Ki-67 ≥ 20%) developed in a quadrant other than the initial primary cancer. We were aware of the several limitations of a single center retrospective analysis. Nevertheless, the data reported in this cohort showed excellent local control and survival rates, comparing very favorably with other published reports on HDR interstitial BT for treatment of IBTR with heterogeneous fractionation schemes and doses (Table 4).
Table 4
Overview of relevant publications on salvage BT
| Reference | Number of patients | Treatment modality | Follow-up | Clinical results |
|---|---|---|---|---|
| Resch et al. [26] | 17 | 8 pts, EBRT 12-30 Gy + PDR boost 12.5-28 Gy 9 pts, PDR 40.2-50 Gy | 59 months (range, 20-84 months) | LC, 70.5% |
| Niehoff et al. [27] | 32 (13 patients post-mastectomy) | 15 HDR, mean dose, 28 Gy 17 PDR, mean dose, 30 Gy | 19 months | LC, 20 pts M+, 32 pts |
| Chadha et al. [28] | 15 | LDR, total dose, 30-45 Gy | 36 months | 3-year OS, 100% MFS, 89% |
| Trombetta et al. [29] | 21 | LDR, total dose, 45-55.3 Gy | 40 months (range, 3-69) | LC, 95.2% OS, 90.4% |
| Polgar et al. [30] | 12 | HDR, 22 Gy; 5 fractions b.i.d. | 56 months (range, 8-112) | MFS, 100% |
| Adkinson et al. [31] | 11 (4 BCa; 6 HD; 1 STS) | HDR, 34 Gy; 10 fractions b.i.d. | 53.7 months | LC, 100% OS, 100% |
| Hannoun-Levi et al. [33] | 42 | HDR 34 Gy; 10 fractions b.i.d. | 21 months | LC, 97% |
| Trombetta et al. [34] | 18 (16 BCa; 2 HD) | HDR (balloon) 34 Gy; 10 fractions b.i.d. | 39.6 months (range, 15-74) | LC, 89% |
| Kauer-Dorner et al. [35] | 39 | PDR, mean total dose, 50.1 Gy | 57 months | 5-year LC, 93% 5-year OS, 87% 5-year DFS, 77% |
| Guix et al. [36] | 48 | HDR 30 Gy; 12 fractions b.i.d. | – | LC at 17 year, 84.2% DFS, 65.4% OS, 90.7% |
| Hannoun-Levi et al. GEC-ESTRO Breast Cancer working group [37] | 217 | HDR (47%) PDR (40.6%) LDR (12.4%) | 46.8 months (range 13.2-123.6) | 5-year LC, 94.4% 10-year LC, 92.8% 5-year OS, 88.7% 10-year OS, 76.4% 5-year M+, 9.6% 10-year M+, 19.1% |
| Cozzi et al. [38] | 40 | HDR, 32 Gy; 8 fractions b.i.d. 34 Gy;10 fractions b.i.d. 16 Gy; single fraction | 61.5 months (range, 6-153) | 5-year OS, 85.3% 5-year LC, 96.6% 5-year M+, 6% |
| Smanyko et al. [39] | 39 | HDR, 22 Gy; 5 fractions b.i.d. | 59 months (range, 1-189) | 5-year LC, 94% 5-year DFS, 69% 5-year OS, 81% |
| Forster et al. [40] | 19 | HDR (58%) 34.2 Gy; 9 fractions b.i.d. 32 Gy; 8 fractions b.i.d. PDR (42%) total dose, 49.8-50.4 Gy | 66 months (range, 18-120) | 5-year LC, 100% 5-year DFS, 100% 5-year OS, 100% |
| Our study | 31 | HDR, 34 Gy; 10 fractions b.i.d. | 73.7 months (range, 28.8-102.4) | 5-year LC, 90.3% 5-year DFS, 83.9% 5-year OS, 87.1% |
[i] BCa – breast cancer, b.i.d. – bis in die, DFS – disease-free survival, GEC-ESTRO – Groupe Européen de Curiethérapie and European SocieTy for Radiotherapy and Oncology, HD – Hodgkin disease, HDR – high-dose-rate, LC – local control, LDR – low-dose-rate, MFS – mastectomy-free survival, M+ – systemic progression, OS – overall survival, PDR – pulsed-dose-rate, STS – soft tissue sarcoma
Niehoff et al. reported on 32 consecutive patients, who were affected by local recurrence after mastectomy (13 patients) or breast-conserving surgery (19 patients) and full-dose adjuvant EBRT. Reirradiation was performed with HDR (15 patients; mean dose, 28 Gy; 2 × 2.5 Gy/day, with 6 hours interfractional interval daily) or PDR-BT (17 patients; mean dose, 30 Gy; 5 × 1 Gy/day, with 2 hours of pulse intervals). After a mean follow-up of 19 months, local control was achieved in 20 patients, but 20 of the 32 patients experienced an additional systemic progression [27].
Guix et al. reported on 36 patients with local recurrence after conservative treatment for breast cancer, treated by a second lumpectomy, and followed by adjuvant HDR-BT (30 Gy in 12 fractions in 5 days). Actuarial LC, DFS, and OS at 10 years were 89.4%, 64.4%, and 96.7%, respectively [32]. In 2011, the same group published an update on 85 patients with breast-only recurrence: 48 patients were treated by a second lumpectomy followed by adjuvant HDR-BT, whereas 37 patients treated by mastectomy had no further radiotherapy. Actuarial LC, DFS, and OS at 17-year were 84.2%, 71.7%, and 65.4% for SBCS, and 63.8%, 90.7%, and 88.2% for mastectomy, respectively. Authors concluded that salvage lumpectomy and BT could be considered the treatment of choice in patients with recurrent breast tumors [36].
Hannoun-Levi et al. presented clinical results of second conservative treatment of combining surgery and HDR-BT (dose per fraction, 3.4 Gy; total dose, 34 Gy) in 42 patients affected by ipsilateral breast cancer recurrence. After a median follow-up of 21 months, second local control rate was 97% with acceptable toxicity and high patient satisfaction regarding cosmetic results [33].
The Groupe Européen de Curiethérapie (GEC) and the European Society for Radiotherapy and Oncology (ESTRO) working group published a retrospective study on 217 patients treated with lumpectomy and multi-catheter HDR (47%), PDR (40.6%), and LDR (12.4%) BT in 8 institutions. After a median follow-up of 3.9 years (range, 1.1-10.3 years), 5- and 10-year actuarial LC, OS, and distant metastasis rates were 94.4% (90.5-98.5%) and 92.8% (87.9-87.9%), 88.7% and 76.4%, 9.6% and 19.1%, respectively, with excellent/good cosmetic result in 85% of cases [37].
Cozzi et al. evaluated a dataset of 40 patients treated with tumorectomy and interstitial intra- or post-operative HDR-BT with different schemes (32 Gy in 8 fractions; 34 Gy in 10 fractions; 16 Gy in single fraction). Late fibrosis > grade 3 was observed in 14 patients (35%). The 3- and 5-year OS was 97 and 85.3%, respectively. Local relapse and metastasis-free survival at 5 years were 96.6% and 94%, respectively [38].
More recently, Smanyko et al. reported clinical outcomes of 195 patients with ipsilateral breast tumor recurrences treated with SBCS with interstitial BT (39 patients; dose per fraction, 4.4 Gy; total fractions, 5; total dose, 22 Gy) or salvage mastectomy (156 patients). After a median follow-up of 6 years, a new local recurrence occurred in 10.2% and 17.9%, and the 5-year disease-free survival was 69% and 65% in the conservative surgery + BT group and in the mastectomy group, respectively. The 5-year probability of overall survival was 81% vs. 66%, respectively [39].
Forster et al. analyzed results of 19 consecutive patients with small, low-risk breast recurrence (rpT1 cN0 cM0, Her2 negative, preferably positive hormone receptor status), treated with SBCS and interstitial multicatheter BT (8 PDR, 11 HDR). After a median follow-up of 65 months, 5-year DFS and OS rates were both 100%; only one patient had a second in-breast recurrence at 77 months after salvage treatment [40].
Comparable results were showed in smaller BT patient cohorts [26,30,31,34] and in patients treated with other types of adjuvant irradiation. SBCS and IORT presented LC and OS of 91-100% and 82-94%, respectively [41,42,43,44,45,46], whereas a second EBRT after SBCS provided LC of 76.9-100% and OS of 62.5-100% [49,50,51,52,53].
Conclusions
Despite small sample size, our preliminary results are comparable with literature data related to good feasibility of the technique, low toxicity profile, good tumor control, and encouraging cosmetic results. A close interdisciplinary collaboration between the surgical and radiation oncology communities is required to individualize each treatment and to maximize patient care.
Accelerated partial breast reirradiation using HDR-BT offers an alternative to mastectomy in carefully selected patients with a favorable breast recurrence. Further clinical trials are needed to identify possible subgroups of patients that might be suitable for this type of approach.