Purpose
With a widespread use of prostate specific antigen (PSA) testing, there has been a strong migration of clinical risk categories towards earlier stages. In Sweden, between 1998 and 2011, there was a two-fold increase in the proportion detected of low-risk prostate cancer from 14% to 28% [1]. Therefore, more men have been considered for curative local treatment of prostate cancer (PC). Available options for local treatment include prostatectomy, radiotherapy, or active monitoring. All methods have pros and cons, and patient preferences have to be taken into account. In a survey from England conducted between 1997 and 2006 with patients with low- and intermediate-risk PC, 40% of them chose surgery (motivation was physical removal), 31% conformal external beam radiotherapy (EBRT) (motivation was fear of other treatments), 21% brachytherapy (motivation was convenient for lifestyle), and 8% of patients selected active surveillance [2]. Low-dose-rate (LDR) brachytherapy with permanent seeds has been used as monotherapy since many years. Long-term (10 years) PSA relapse-free survival results with modern seed technique was 86-95% for low-risk and 80-90% for intermediate-risk PC in three large series with long follow-ups [3-5]. Based on the assumption of a low α/β ratio for PC, high-dose-rate (HDR) brachytherapy with extreme hypofractionation was proposed [6]. In theory, the radiation effect on PC cell would increase, and side effects from organs at risk (OARs) would decrease. Several publications show a low α/β ratio from 1.4 to 2.6 [7], but the ratio might be heterogeneous and uncertain. First clinical series on HDR monotherapy were published in 2010-2011, with excellent biochemical control rates of 85-97% after 5-years median follow-up [8, 9]. Physical dosimetry with respect to the target and OARs has recently been proved to be better with HDR compared to LDR monotherapy in a randomized study [10]. Moreover, side effects from the treatment seem to be milder with HDR compared to LDR seed therapy [11]. HDR monotherapy was introduced systematically at the Örebro University Hospital in 2004. The aim of this retrospective study was to evaluate treatment results regarding biochemical control rate, disease-free survival, and side effects after at least 5-years of follow-up.
Material and methods
Between March 2004 and April 2012, we consecutively treated 231 men with biopsy-proven PC. Our inclusion criteria were T1c-T2cNxM0 PC with PSA ≤ 15 if Gleason ≤ 6 (low-risk group), or T1c-T2bNxM0, PSA ≤ 10 if Gleason 3 + 4 (intermediate-risk group). No patient was lost for follow-up. A written consent was obtained from every patient, except two (both are alive with no evidence of disease). Characteristics of the evaluated 229 patients are presented in Table 1.
Table 1
Patient characteristics in the three different fractionation cohorts
Diagnostic work-up
All patients had a digital rectal examination and a trans-rectal ultrasound (TRUS) with 6, and later (after 2006) with 8 biopsies. Bone scan, computer tomography (CT), or magnetic resonance imaging (MRI) were only performed in various cases. The patients were classified into risk groups according to the Memorial Sloan-Kettering definition [12]. Pre-treatment PSA, prostate volume (from TRUS), and Gleason score was registered. Symptoms scoring before the treatment and prospectively during follow-up regarding lower urinary tract symptoms (LUTS) were defined as:
no: 0-1 nocturia, no urgency/dysuria (G0),
mild: 2-3 nocturia, single urgency/dysuria (G1),
moderate: 3-4 nocturia, sporadic urgency/dysuria; need of medication (G2),
severe: > 4 nocturia, severe urgency; need of temporary catheterization (G3),
permanent: permanent catheter or corresponding solution (G4).
Bowel symptoms were defined as:
no: 1-2 normal defecations daily (G0),
mild: 2-4 defecations daily; single urgency/bloody stools (G1),
moderate: > 4 defecations daily; frequent urgency/bloody stools; need of medication (G2),
severe: continuous struggle; need of surgical treatment (G3).
Erectile dysfunctions (ED) were defined as:
no ED: normal erectile function,
moderate ED: weak or no erection,
complete ED: total loss of erectile function.
Additionally, a self-assessment using international prostate symptom score (IPSS) and international index of erectile function score (IIEF) was assessed.
Radiobiology and fractionation schedules
Our motivation to start HDR as monotherapy in 2004 was based on three components: radiobiology findings with low α/β ratio for PC, a new three-dimensional (3D) TRUS real-time intra-operative dose planning system (Swift planning system, Nucletron BV, Veenendaal, The Netherlands), and early treatment experience from US [13] and Germany [14]. In the beginning, we decided to use a 9.5 Gy × 4 fractions schedule over 2 days (4F cohort). The patients had to stay in bed laying on the back, with the plastic needles fixed by sutures to the perineum. We discovered several logistic problems in that treatment, such as oedema of the prostate target and movement of the catheters between fraction 1 and 2, urging for supplementary dose plan and catheter adjustment. Pain and discomfort of the patients required both spinal and epidural anesthesia. All the above were the reasons why we early moved to two or three separate implants with steel needles to have a quicker and more accurate dose planning. Iso-effective schedules with a linear quadratic formula and an assumed α/β ratio = 3 were calculated. The equivalent dose 2 Gy (EQD2) = 95 Gy for both 9.5 Gy × 4/48 hours and 14 Gy × 2/2 weeks schedule, and EQD2 = 92 Gy for the 11 Gy × 3/4 weeks schedule. For pragmatic reasons, we choose to use the 11 Gy × 3 schedule for the younger (≤ 65 years) cohort and the 14 Gy × 2 schedule for the elderly (≥ 66 years).
Treatment procedure
The patient was prepared with a small rectal enema in the evening before and in the morning of the implant day. Prophylactic ciprofloxacin was administered on the implant day. The patient had a urethral catheter during the procedure, that was left in place until the day after implantation. All implants’ procedures were performed in the lithotomy position. The vast majority of the procedures were done with spinal anesthesia, although few patients had general and some epidural anesthesia in the 4F cohort.
The TRUS probe was applied and adjusted with the aid of a water standoff distance from the probe tip to let the posterior border of the gland fit to the posterior needle row on the perineal template. Two anchor needles were inserted one on each side of the urethra to hold the gland in a stable position.
With a cranial to caudal movement of the TRUS probe, a US-tomography with 1 mm slices were obtained (pre-planning scan). In the dose planning system Swift® and later, Oncentra Prostate® (Nucletron BV, Elekta AB, Stockholm, Sweden), the slices were converted into a 3D volume. The clinical target volume (CTV) and OARs were delineated in every 5 mm slice. The urethra was defined as the outer surface of the urethral catheter (7 mm diameter), and the whole thickness of the rectal wall and rectal mucosa was delineated 5 mm beyond the base and apex plane. In the last part of the pre-planning phase, virtual needles were placed in the outer ring just inside the largest section of the prostate, and the inner ring of the needles not too close to the urethra, to deliver central dose and aid a conformal dose at the apex and base plane.
The implant was performed with needles, median numbers, 18 (range, 16-23) adjusted to the same insertion depth. Finally, we performed a new US tomography (live scan) with the needle implant, and adjusted all structures according to position of the implant. In the final dose plan, all the changes in prostate volume (elongation, oedema), movement of OARs, and deviating needles were considered, and the dose distribution was optimized accordingly. Because the CTV was delineated as the prostate capsule without any margin, there was a high requirement to cover the CTV. The relative clinical target volume having 100% of the prescribed dose, V100, CTV ≥ 97.5%, and the relative dose constraints to the urethra and rectal mucosa were Dmax, urethra ≤ 110% and D10, rectal mucosa ≤ 65%. The details of our dose planning and dwell positioning strategies performed were published elsewhere [15].
Finally, MicroSelectron HDR afterloader (Elekta AB, Stockholm, Sweden) administered the treatment and the implant was extracted.
Follow-up
Prostate specific antigen was monitored every 6 months, up to 3 years, and then annually. A nurse contacted every patient over the phone at these occasions. As described before, relevant symptoms were recorded and scored according to EORTC common toxicity criteria (CTC version 2.0). When indicated, the patient could have an appointment with a radiation oncologist, a urologist, or a radiology examination. A patient-reported questionnaire regarding IPSS and IIEF was collected at baseline, and 3-, 5-, and 10-years of follow-up.
Statistics
Data from a clinical database was retrieved and analyzed. Biochemical (PSA) decline, PSA failure according to ASTRO definition [16], and side effects were descriptively evaluated. Disease-free survival (DFS) and freedom from PSA failure were analyzed with Kaplan-Meier method. Log-rank test and Cox regression analyses with and without age adjustment were performed to assess any differences between various fractionation schedules and factors related to PSA failure. Wilcoxon signed-rank test, Pearson’s χ2 test and paired t-test were used to analyze side effects.
Results
PSA regression and PSA failure rate
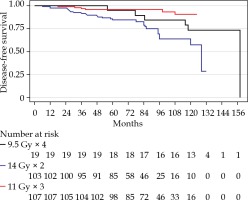
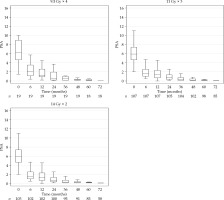
After a median follow-up for the whole cohort of 7.1 years, PSA failures were found in the total of 22/229 (9.6%), 2/19 (10.5%) in the 4F group, 5/107 (4.7%) in the 3F group, and 15/103 (14.6%) in the 2F group. The distribution between risk groups were 9 with low-risk (5%) and 13 with intermediate-risk (22%). Figure 1 shows a Kaplan-Meier plot of DFS for the three cohorts. Figure 2 presents similar PSA regression for the three different cohorts. PSA values at and after PSA failure were excluded from the analyses. A PSA nadir < 1 µg/l was reached after two years in all fractionation cohorts.
Fig. 2
Box plot of prostate specific antigen (PSA) during follow-up showing similar regression for three different fractionation cohorts
Prostate specific antigen occasionally bounced after the treatment and occurred between 12th and 24th month. It was more frequent in the 4F (26%) and 3F (14%) groups than the 2F cohort (8%). Figure 3 demonstrates freedom from PSA failure for the three cohorts. Figure 4 and Table 2 show freedom from PSA failure (bFFF) for the 2F and 3F cohorts, with respect to low- or intermediate-risk groups.
Fig. 3
Kaplan Meier plot of freedom from prostate specific antigen (PSA) failure for three different fractionation cohorts
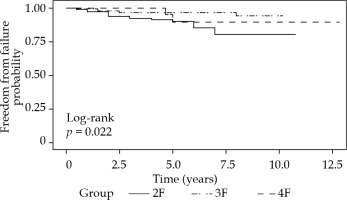
Fig. 4
Kaplan-Meier plot of freedom from prostate specific antigen (PSA) failure for low- and intermediate-risk groups
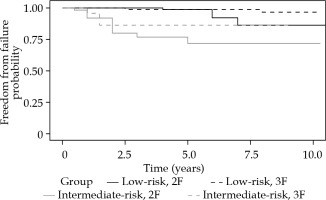
Table 2
Freedom from prostate specific antigen (PSA) failure at 5 and 7 years from Kaplan-Meier calculation
| Risk group | All 4F | All 3F | All 2F | LR 3F | LR 2F | IR 3F | IR 2F |
|---|---|---|---|---|---|---|---|
| 5-year | 89% | 96% | 90% | 99% | 98% | 86% | 72% |
| 7-year | 89% | 96% | 85% | 99% | 92% | 86% | 72% |
Log-rank test showed a significant difference between the groups (p = 0.02), where 3F (reference) resulted better than the 4F and 2F groups. When performing a Cox regression and adjusting for age (cut-off < 66 years), the fractionation was no longer significant (p = 0.81). Only the 2F and 3F groups were included in Cox regression analysis. Even though the 4F group was too small for this evaluation, this group did not stand out, and we could exclude it without risking incorrect conclusions (as shown in the graph).
We tried to find factors predicting PSA failure using log-rank test, and found initial PSA (cut-off ≤ 10) (p < 0.001) and age (p = 0.004) to be of significant importance. On the other hand, T-stage (cut-off between T1 and T2) was on borderline (p = 0.06) and Gleason (cut-off between 3 + 3 and 3 + 4) score was not significant (p = 0.75). When performing Cox regression, including the three significant (or nearly significant) parameters, all of them were still significant (age, p = 0.02; initial PSA, p < 0.001; T-stage, p = 0.03).
Disease-free survival
In total, 23 of 229 patients died during the follow-up period, and only four patients were related to PC cause. The vast majority died in the 2F elderly group, and the cause of death was non-cancer or unknown causes. We performed Cox regression analysis of disease-free survival and found no significant difference between different fractionation after age adjustment (p = 0.5 for the 4F group, and p = 0.15 for the 2F group).
Treatment side effects
Lower urinary tract symptoms (LUTS)
The baseline and endpoint data are presented in Figure 5. In this figure, the maximum grade of LUTS during the follow-up is visible, which reflect acute and transient side effects. The fraction of men without LUTS (baseline compared to endpoint) slightly reduced, but there was a trend towards more severe problems. Severe late urinary toxicity, G3 and G4, was limited to 3.5%, and included one G4 (4F) case of urostomy after a fistulation, one G4 (2F) case with a stricture treated by bladder neck incision, and one (3F) case classified as G4 (by intent to treat) due to a cystoprostatectomy from muscle invasive bladder cancer appearing 3 years after brachytherapy. There were 5 G3 cases, with 3 permanent and 2 intermittent catheterizations.
Fig. 5
Prevalence of baseline maximum and endpoint lower urinary tract symptoms (LUTS) for all patients
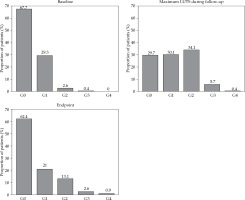
When considering individual LUTS changes during the follow-up, 55.5% of the whole study population reported no change compared to baseline. A remaining minor impairment, defined as one-step in the scale above baseline, occurred in 20% of the patients, and a major impairment (two steps or more) occurred in 10%. However, among men with unchanged status, about half of them experienced a temporary deterioration (defined as temporary increased problems at some point during the follow-up), which was likely to reflect an acute but transient side effect. Pearson’s χ2 test was performed to evaluate any differences between 2F and 3F cohorts. Temporary impairment was seen in both cohorts in 31% of the patients (p = N.S.). Remaining impairment was more common (37%) in the 2F group than the 3F cohort (22%) (p = 0.03). Unchanged LUTS was more common in the 3F (36%) compared to the 2F cohort (18%) (p = 0.005). Differences between the 2F and 3F cohorts seemed to be more dependent on age than fractionation.
In this study, 169 of the participants also completed IPSS questionnaires at the baselines and during follow-ups (at 3-, 5-, and 10-years). The possible outcomes of these questionnaires ranged from 0 to 35. Analyzing the difference between the measures from each patient showed an approximately normal distributed outcome, with mean value of 1.4 and standard deviation of 6.6. This means that the patients on average scores presented with 1.4 points higher at the second occasion. This increase reflects a slight impairment that was significantly (p = 0.006) different from zero, but since there was no control group, we cannot confirm whether this was due to the treatment or aging effects. In a sub-group analysis, the impairment was no longer significant, when only the 3F group composed by younger men was taken into account.
Erectile dysfunction
Considering only the initially fully potent men, we could observe that they developed ED, in some extent, in 77% of the cases, but only 21% had complete ED at the endpoint. Among men with moderate ED at the baseline, about half of them remained at the same level, and the other half developed a complete ED.
In a sub-group analysis performed with Pearson’s χ2 test, we first considered men without initial ED comparing the 3F and the 2F cohorts, where 28% vs. 13% remained with no ED (p = 0.08), 62% vs. 49% declined to moderate ED (p = N.S.), and finally, 10% vs. 38% ended up with complete ED (p = 0.0004).
When we consider men with initial moderate ED in the same sub-group analysis, there were no significant differences between the 3F and 2F groups. About 50% of them remained with moderate ED, and the other half declined to complete ED.
Discussion
There is a growing body of long-term experience in HDR monotherapy in literature. Table 3 summarizes published studies with high patient number (> 100) and long follow-up (> 5 years) [17-22]. Long-term biochemical control rate depends on follow-up time, patients’ selection, precision of diagnostic work-up, and adjuvant use of androgen deprivation therapy (ADT). The present study confirms results from other centers. We did not use ADT in our study that might explain lower biochemical control rate in intermediate-risk PC patients. The use of ADT is probably more important in intermediate-risk and necessary in high-risk cases. When comparing our results to other series, we used the ASTRO definition of PSA relapse. If the Phoenix definition (nadir +2) [23] had been used in our study, PSA relapse events occurred in mean 16 months (range, 0-60 months) later. During the time of the study, staging procedure was very basic with digital examination and 6 to 8 systematic biopsies. Studies made with clinical and following post-operative staging shows upgrading and upstaging in more than 33% of low- and intermediate-risk PC. These findings correlate with age, PSA level, and PSA density > 0.15 [24, 25]. Therefore, this might explain our worse results in the elderly intermediate-risk group. One patient in the 2F intermediate-risk group included in the analysis had bone metastases during the treatment that was not noticed by the referring hospital. We had 22% of PSA failure (ASTRO definition) at 5 years for the intermediate-risk group without adjuvant ADT, which was similar to a randomized HYPO-RT-PC study with 16% (Phoenix definition) [26].
Table 3
Published long-term (≥ 5 years) results
| Centre | n | D/fx. | FU | bNED L/I/H | ADT | Ref. |
|---|---|---|---|---|---|---|
| Osaka, JPN | 190 | 45.5-54/7-9 | 7.6 | –/93/81 | 73 | [17] |
| Los Angeles, USA | 448 | 42-43.5/6 | 6.5 | 99/95/– | 9 | [18] |
| Offenbach, GE | 492 | 38/4 | 5-7.7 | 95/93/93 | 19-21 | [19] |
| Detroit, USA | 319 | 38/4 | 5.5 | 98/98/– | 19 | [20] |
| Northwood, UK | 244 | 26-31.5/2-3 | 5-9 | –/93-91/93-91 | 76-87 | [21] |
| Lecco, IT | 277 | 38-27-19/4-2-1 | 6 | 81%* | 34 | [22] |
| Present study | 229 | 38-33-28/4-3-2 | 10-7-6 | 95/78/– | 0 |
[i] n – number of patients, D – total dose (Gy), fx. – number of fractions, FU – follow-up (years), bNED – PSA control rate (%), L – low-risk, I – intermediate-risk, H – high-risk, ADT – proportion with the use of androgen deprivation therapy (%), * PSA progression-free survival, 91% for 4 fx., 86% for 2 fx., and 65% for 1 fx.
Prostate specific antigen regression between the different fractionation cohorts was nearly identical, supporting a low α/β ratio of 3. In a recent radiobiological study, based on overview data from clinical studies with few and high doses per fraction, the calculated α/β ratio was surprisingly 22.8 Gy, suggesting that the value was uncertain with high and few fractions [27]. In contrast, other published calculations of α/β ratio showed a low value of 1.4 [7]. This reflects uncertainties, and α/β values might be different for high and low differentiated PC cells, which was suggested in a simulated calculation of LQ model extension (DVIC-BED) [28].
It is difficult to make a comparison with prostatectomy, but in a systematic review [29], the authors found a biochemical control rate (PSA < 0.2) at 7 years of 80%. This review summarizes results from studies with all kind of clinical and pathological risk groups. It is interesting to see that HDR monotherapy and LDR seed monotherapy compares well to surgical results. This was also confirmed in a comparative analysis of PSA-free survival from the Prostate Cancer Results Study Group [30]. Moreover, biochemical relapse-free survival (BRFS) at 3 years/7 years was 97%/94% for low-risk PC and 94%/83% for intermediate-risk PC, respectively, in a large multicentric Swiss study on 125I LDR brachytherapy [5]. The authors found PSA > 10 and Gleason > 6 to have a significant worse BRFS outcomes. This was similar to our data.
In the present study, we tried to analyze the longitudinal side effects from the treatment, and we could observe a temporary 1-2 grades increase of LUTS during acute reaction. The acute urethral grade 2-3 toxicity of 24% compares well to the large Offenbach series, with 20-25% of grade 2-3 reactions [19]. Only 3% of patients had a temporary catheterization, which was less then reported from LDR seed brachytherapy series [27]. When considering late LUTS effects, we found no or minor impairments. Severe grade 3-4 late toxicity of 3.5% compares well to published results mentioned in Table 2, with 1-11% of grade 3 late urinary toxicity [31, 32]. LUTS is common even in an untreated old male population, and the patients are on average 7 years older at endpoint compared to baseline. Since this was not a randomized study, it was difficult to determine whether these were real treatments’ side effects or just an age effects. However, the transient impairment was probably related to the treatment.
Bowel side effects were none or mild in a few cases in this study, which corresponds to published data with 0-1% of grade 3 rectal toxicity [31]. This was obviously better than EBRT, where moderate-severe symptoms occurred in 5-15% of patients [26, 33].
The erectile function significantly declined, and this could be classified as a side effect of the treatment, but at the same time, it could be due to aging during the follow-up period. In this study, the mean age at the baseline was 64 years and at the endpoint 71 years. Long-term complete ED was 21% for men with normal erectile function before the treatment, which was similar to the Offenbach data [19]. This was also similar to the report from the William Beaumont Hospital, where HDR brachytherapy was found to be more favorable than LDR seed brachytherapy regarding erectile dysfunction [11]. Increasing age during follow-up is in itself a confounding factor to distinguish LUTS and ED from the treatment versus ageing [34, 35].
In a retrospective study, there are certain limitations, such as selection bias and lack of a control group. All our patients were consecutively included, and had a prospectively complete long follow-up regarding PSA and treatment side effects. No patients were lost for follow-up. The biochemical control rate was lower in the elderly 2F intermediate-risk group, which might be due to not using adjuvant ADT or incorrect staging. The PSA regression and side effects of the different fractionation cohorts were similar, because since 2015, we only using two fractions of 14 Gy for all patients with indication for HDR monotherapy.
In conclusion, this retrospective study with 7-years median follow-up showed 95% and 78% biochemical control rate in clinically staged low- and intermediate-risk prostate cancer patients, respectively. Overall, the early and late side effects from the treatment were mild.