Purpose
Primary cutaneous lymphomas (PCLs) are a very heterogeneous and relatively rare group of diseases, with an estimated annual incidence of 0.7/100,000 citizens [1]. They differ from other lymphomas, as they occur only in the skin without invading other organs and tissues. PCLs are divided into two main subgroups: cutaneous T-cell lymphomas (CTCLs) and cutaneous B-cell lymphomas (CBCLs). The latter is less common and represents less than 25% of all PCLs. CBCLs contain primary cutaneous follicle center lymphomas (PCFCLs), primary cutaneous marginal zone B-cell lymphomas (PCMZLs) about the indolent course, primary cutaneous diffuse large B-cell lymphomas, leg type (PCLBCLs LT), and primary cutaneous diffuse large B-cell lymphoma, other types (PCLBCLs OT) whose course is usually more aggressive [2,3].
According to the most important recommendations for CBCLs treatment, in all their subtypes and locally advanced cases, a treatment with ionizing radiation with external beam radiation therapy (EBRT) methods is recommended [4,5,6,7]. Even though high-dose-rate brachytherapy (HDR-BT) is a radiation technique enabling delivery of higher doses more precisely than EBRT [8], and results in excellent treatment outcomes for other skin cancer types [9,10], none of those above-mentioned recommendations indicate BT as a treatment option for CBCLs. It is probably due to only a few reports in literature, which directly correspond to PCLs treatment using HDR-BT in palliative setting and for lesions derived from T-cell lines [11,12]. However, there are no reports on HDR-BT exclusively dedicated to CBCLs. The purpose of this paper was to report the first case series of CBCLs treated with HDR-BT, and to initiate filling the gap in the literature.
Material and methods
There were 7 patients treated between 2011 and 2019 (6 males and 1 female), with 12 skin lesions histopathologically proven as CBCLs (5 cases of PCMZL, 4 cases of PCLBCL OT, 2 cases of PCFCL, 1 case of PCLBCL LT). All patients were referred for BT treatment after a complete assessment performed by a hematologist. The mean age at the time of presentation was 53.3 years (median, 55.4; range, 34.4-72.2). Staging was assessed according to TNM classification, determined by the International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (Table 1) [13], and included T1a and T2a in 4 and 8 of treated lesions, respectively. However, the clinical manifestation of some lesions did not correlate thoroughly with TNM classification: in 4 cases, multi-nodular changes were described (clear T2a), in 4 cases – solitary nodules (clear T1a), but four tumors (two in two patients), which co-occurred in the skin area of a diameter less than 15 cm (T2a) were treated as solitary tumors, as they were located in different anatomical sites (cheek and forehead; left and right lumbar area).
Table 1
ISCL/EORTC proposal on TNM classification of cutaneous lymphoma other than mycosis fungoides and Sézary syndrome [13]
HDR-BT was prescribed as the first-line treatment for all studied cases, as the second-line treatment for recurrences after surgical failure for 4 cases, and as an adjuvant treatment after insufficient surgery for 1 patient. Additionally, it is to be noted that 5 out of the tumors mentioned above were treated as new consecutive lesions that appeared in time in 2 patients, who were treated earlier in our institute. Moreover, three quarters of treated lesions were staged as secondary according to the TNM classification (feature rT, recurrent tumor).
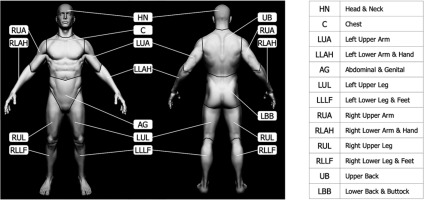
According to TNM location indicators, the lesions were located as follows: head and neck (HN) 59%, lower back and buttock (LBB) 25%, upper back (UB) 8%, and left lower arm and hand (LLAH) 8% (Figure 1) [13].
Fig. 1
Visual representation of skin surface regions of a human body according to the TNM classification [13] (the figure is based on the anatomical model by Stefan Polster, available at https://www.artstation.com/artwork/X28Yl)
All tumors were treated with MicroSelectron HDR afterloader (Nucletron, an ELEKTA company, Stockholm, Sweden) using 192Ir stepping source, with nominal activity 10 Ci. Out of 12 lesions, one was treated interstitially (6 single-leader applicators) due to the thickness of infiltration well above 5 mm and was planned three-dimensionally. The remaining eleven tumors were treated superficially with Freiburg-flap applicators and planned in 3D (3 lesions) or in 2D setting (8 lesions). As for 3D planning, patients’ anatomies and application reconstructions as well as dose-volume calculations were completed with Oncentra Brachy software (Nucletron, an ELEKTA company, Stockholm, Sweden).
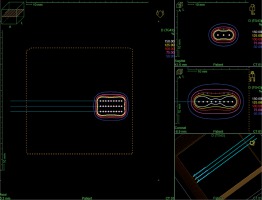
In cases of two-dimensional planning, precise measurements of tumors and patients’ skin marking were performed by a dedicated physician. The Freiburg-flap applicator size was adjusted to cover the tumor with a 5 mm margin in all directions. 2D treatment plans were prepared without imaging. In the phantom, a physicist created parallel catheters using the same amount as the number of channels in a real applicator, separated by 1 cm. Source positions located above the lesion plus margins were activated. A standard step size of the source was set at 5 mm. Subsequently, parallel axis dose points were made for each active source position at a distance of 10 mm. Dose distribution was normalized to these points and then, a plan was optimized using dose point optimization with a distance option and dwell-time gradient ratio (DTGR) of 0.3. In such a plan, 100% isodose was located 5 mm beneath the skin surface and intersects the patient’s skin at a distance of 3 to 5 mm from the applicator (Figure 2).
Fig. 2
Reconstruction of 2D planned application in a TPS phantom. Optimization dose points located at 10 mm from the active dwell positions in the plane parallel to the applicator
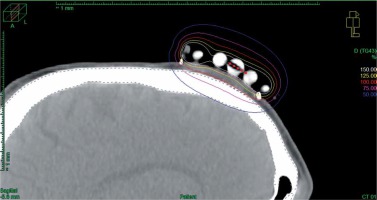
In cases of three-dimensional planning for superficial applications, after the applicator was placed and secured on a tumor and the skin was marked appropriately, computed tomography (CT) with a 3 mm slice thickness was performed. Next, dedicated physician delineated clinical target volume (CTV) and organs at risk (OARs), and the physicist reconstructed the applicator digitally. The source positions above the CTV were activated to plan an optimal dose distribution. There was no margin added to CTV. Then, axis dose points were combined and adapted to the thickness of the lesion, or the applicator points were added manually and adapted to deeper surface of the lesion. Dose distribution was normalized to the defined points and then optimized using dose point optimization with a distance option. Additionally, the dose distribution with graphical optimization was adjusted for every patient (Figure 3).
Fig. 3
Sagittal CT reconstruction of 3D planned contact application for tumor localized on the scalp of the patient. 100% isodose is shaped to cover the target and avoid the skull
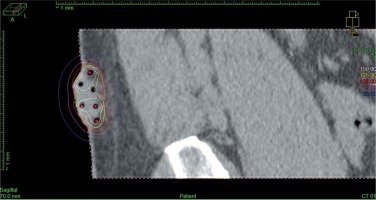
In a single case of interstitial application, CT imaging was done with a slice thickness of 2 mm. Once the CTV and OARs were delineated, interstitial applicators were digitally reconstructed. No markers were used to avoid artifacts, as the air in the applicators’ lumen contrasted sufficiently well with surrounding tissues. The step size of the source was set at 2.5 mm. Dose distribution was optimized with inverse planning simulated annealing (IPSA), and source positions were activated inside the CTV. Again, DTGR of 0.3 was used. The final dose distribution was manually adjusted with graphical optimization (Figure 4).
Fig. 4
Sagittal CT reconstruction of interstitial application with 6 catheters. The treated region is clearly visible in CT images. Dose distribution was optimized graphically to cover the target
Median planned physical dose (PD) was 36 Gy (range, 30-40 Gy) in 10 fractions (range, 6-10 fractions), with median overall treatment time (OTT) of 11 days (range, 4-11 days). The most frequent fractionation pattern was ten times 3.6 Gy (OTT, 11 days), which was used to treat seven lesions. The used regimens differed due to three factors, including the lack of recommended doses for HDR-BT modality, patients’ convenience, and their possibility to commute for the treatment.
To compare different fractionation patterns, PDs were calculated to biologically effective doses (BED) and the equivalent total dose in 2 Gy per fraction (EQD2) both for CBCLs’ cells and early toxicity (α/β = 10 Gy) and for late effects (α/β = 3 Gy) [14,15]. The equation used for calculations was as follows:
BED = nd [1 + d/(α/β)] and EQD2 = BED / [1 + 2(α/β)],
where n – number of fractions, d – fraction dose, α/β – the ratio of coefficients determining the death of cells due to the passage of one or more radiation quanta.
The cancerous cells’ repopulation factor was not included in the calculations because of short OTTs (< 14 days).
The first control visit was scheduled four weeks after BT completion, and subsequent assessments were at intervals of 2 to 6 months. Treatment toxicity was evaluated according to the RTOG scale [16]. Early radiation reactions were assessed at the first control visit, late effects were noted after three months and till the end of follow-up. Full patient characteristics, detailed lesions’ and treatment data are presented in Table 2. No statistical analyses were performed due to small patients and tumors numbers.
Table 2
Patients, lesions, and treatment characteristics
[i] ID – identification number, PCLBCL LT – primary cutaneous diffuse large B-cell lymphomas, leg type, PCMZL – primary cutaneous marginal zone B-cell lymphoma, PCLBCL OT – primary cutaneous diffuse large B-cell lymphomas, other type, PCFCL – primary cutaneous follicle center lymphoma, PT – primary treatment, R – recurrence after previous surgery, A – adjuvant after non-radical surgery, HN – head and neck, UB – upper back, LBB – lower back and buttock, LLAH – left lower arm and hand, PD – physical dose, Fx no. – number of fractions, OTT – overall treatment time, *lesions T2a treated as solitary tumors
Results
The mean follow-up for all patients resulted in 41 months (median, 40.2; range, 6-101.2 months). Mean BED for tumor and early reactions was 50.3 Gy (median, 49; range, 39-57.6 Gy), and mean EQD2 for tumor and early reactions was 41.9 Gy (median, 40.8; range, 32.5-48 Gy). With the doses delivered, both the complete remission (CR) rate and local control (LC) rate for all the tumors at the last follow-up visit was 100%. However, the treatment was associated with early skin reactions in all cases. The rates of early reactions at the first control visit were as follows: erythema (G1) 33%, patchy epidermal desquamation (G2) 25%, confluent epidermal desquamation (G3) 25%, and minor bleeding (G4) 17%. Despite the already mentioned fact of recurrences in two patients after the first course of HDR-BT (patients no. 2 and 3), at the last follow-up visit, none of the patients presented signs of active disease.
Mean doses calculated for late reactions included BED – 84 Gy (median, 79.2; range, 60-108 Gy) and EQD2 – 50.4 Gy (median, 47.5; range, 36-64.8 Gy). Skin late effects appeared in the irradiated areas in all cases. Most of them were slight depigmentation, G1 (59%). In one case, there was a small telangiectasia, G2 (8%). However, in one patient (patient no. 3), who was treated for four separate lesions, an increased radiation reaction was observed in all treated sites; in three sites irradiated superficially, massive telangiectasia developed, G3 (25%), in one site irradiated interstitially, small ulceration developed in time, G4 (8%).
Results, toxicity, and calculated doses for lesions are presented in Table 3.
Table 3
Outcomes, toxicities, and biologically effective and equivalent doses of treatment
Discussion
Recommendations for treatment methods for solitary or localized CBCLs vary depending on their histopathological type, and include surgery (only very well limited PCMZLs and PCFCLs), systemic treatment (as first-line treatment in case of PCLBCLs), and radiotherapy (first-line treatment in PCMZLs and PCFCLs, combined with systemic treatment for PCLBCs, or solitary treatment as an alternative for combined treatment) [5]. Recommended forms of radiotherapy consist of electrons (6 to 9 MeV) with boluses (to avoid skin-sparing) or low energy X-rays (approximately 100 kV). In cases of tumors with deep infiltration or located at curved surfaces, it is recommended to use high-energy photon radiation. Recommended EBRT doses range from 24 to 40 Gy in conventional fractionation. The dose should be specified to the primary tumor with a safe margin of 1-2 cm (to the sides and depth) [4,5,6,7]. A cumulative summary of studies concerning EBRT shows excellent efficacy resulting in a 99% CR rate for PCMZLs and PCFCLs, and an 88% CR rate for PCLBCLs [4].
In comparison to the above-mentioned EBRT recommendations, the presented 2 Gy normalized doses in this report, in most of the cases, were slightly higher than 40 Gy (median, 40.8 Gy). However, due to unique aspect of HDR-BT, i.e. steep dose fall-off with squared distance from the radioactive source, dose values on the skin surface and in the tumor were substantially higher, which could potentially influence better therapeutic result. On the other hand, the steep dose fall-off might result in a higher number of marginal recurrences. In the presented group of patients, depending on the planning method, safety margins added to clinically visible tumors were smaller than these recommended for EBRT, and varied from 0 (3D planning) to 5 mm (2D planning), so that the areas covered by 100% isodose ranged from 2.25 to 41.5 cm2 (mean, 19.9 cm2; median, 20 cm2). It means that the median EQD2 at a distance of 1 cm from the treated lesion was in the range between 17.7-28.6 Gy (the margin was covered by isodoses of 50-75% of PD), which was still high enough in the presented group to prevent local recurrences in the irradiated sites.
Interestingly, quite a high percentage of occurred late effects higher than G2 (33% total) was observed. It is higher than reported 8.6% for EBRT results [17]. In our institution, HDR-BT has been used for many years in the treatment of various skin neoplasms, most often basal cell carcinomas, squamous cell carcinomas, and distant skin metastases of different origins. Fractionation regimens used in these cases were more aggressive than for the treatment of CBCLs, and despite that, late effects graded higher than G2 were merely 3.4-11% [18,19].
All high-grade late effects occurred in patient no. 3 only. One case of small ulceration (G4) developed over five years after the completion of interstitial treatment of lesion no. 6 (Table 2). The ulceration occurrence may be partially explained by the high value of BED, calculated for late reactions in the delivered regimen of radiation with short OTT. Additionally, the lesion was located in an old scar after Lyme disease, in a tissue, where the initial regeneration capacity was limited. The patient also indicated to undertake physical activity in a gym, and the exercises, i.e. lying on his back (on a bench) with a load, could be considered as an additional possible damaging factor. Also, other late reactions in the form of massive telangiectasia occurred in areas treated with more extended regimens and lower doses that did not cause such substantial complications in other patients. The same pattern as for lesion number 6, but for a contact treatment was used for another patient to treat lesion number 10, where a delayed reaction of only G1 after almost four years was observed. In our opinion, this information suggests that the occurrence of much worst reactions in patient no. 3 may have been caused by an increased individual sensitivity to ionizing radiation, which is frequently disregarded in clinical practice but has been described in the literature from the very beginning of radiotherapy [20,21].


