Purpose
Extraskeletal myxoid chondrosarcoma (EMC) is an extremely rare sarcoma sub-type that affects less than one per million people annually. It usually occurs in adults, with a median onset in the fifth decade of life [1]. Most EMCs arise in deep soft tissue of the proximal extremities and limb. The most common primary site is the thigh [2, 3]. EMC is commonly perceived as a soft tissue mass, and is accompanied by pain and tenderness. Occasionally, some tumors may mimic a hematoma and when located close to joints, can cause functional impairment. However, definite diagnosis of EMC has been difficult so far. Stenman et al. reported translocation involving nuclear receptor sub-family 4 group A (NR4A3) gene on chromosome 9q31.1 (previously mapped to 9q22) in 1995 for the first time [4]. Recently, positivity for NR4A3 re-arrangements was considered a hallmark of EMC [1]. Standard treatment for localized EMC entails wide local resection similar to treatment for other soft tissue sarcomas (STS) [5-7]. Surgery combined with external beam radiotherapy (EBRT) showed better local control compared to surgery alone [8-10]. Of note, the rate of metastasis after radical surgery in EMC ranges from 25% to 50% [2, 3, 8, 11-14]. Generally, palliative radiotherapy (RT) for metastatic EMC is mainly performed using EBRT, and to the best of our knowledge, no report on the use of brachytherapy (BT) for EMC is available so far. This is the first report on a patient with metastatic EMC, who was treated with high-dose-rate (HDR) interstitial brachytherapy (ISBT).
Case presentation
An 87-year-old woman presented with a slow-growing tumor of the right ankle joint discovered 30 years ago (Supplementary Figure 1). She refused to consult a doctor because she only experienced few symptoms. In 2015, the patient’s ankle joint tumor underwent necrosis with infection (Supplementary Figure 2), and she was diagnosed with EMC of the right ankle joint based on biopsy results. She was subsequently referred to department of musculoskeletal oncology at our hospital. The right ankle joint magnetic resonance imaging (MRI) is shown in Supplementary Figure 3. Chest computed tomography (CT) performed at that juncture revealed solitary lung metastasis. Initial TNM and clinical stage were T2bN0M1 and stage IV (UICC 7th), respectively. The right lower leg was amputated three months after the initial diagnosis (Supplementary Figure 4). Fluorescence in situ hybridization showed translocation of NR4A3 gene, and diagnosis was EMC was histopathologically confirmed. Adjuvant therapy was not administered owing to her advanced age, and follow-up was performed. In 2017, a tumor appeared in her right inguinal region, which gradually increased in size, and was considered to be EMC recurrence (Figure 1A). At 16 months after surgery, CT depicted a 12-cm lymph node metastasis in the right inguinal region and multiple lung metastasis without any symptoms (Figure 1B). She was then referred to department of radiation oncology. Close proximity between any large vessels and the right inguinal tumor was not depicted in ultrasonogram (US). The patient and her family wished to treat the right inguinal tumor to avoid tumor-related symptoms of discomfort (including skin ulcer, bleeding, infection, and edema). However, she could not undergo EBRT every day at the outpatient clinic because of her age; she also refused long-term hospitalization. Therefore, we planned a short-course HDR-ISBT for the right inguinal lymph node metastasis aiming for both palliation and local control. Dose of HDR-ISBT was 30 Gy/2 fractions in 1 day (only one application). We performed US-guided HDR-ISBT with a 5-French ProGuide sharp needle (Elekta, Sweden). During applicator implantation, anesthesia was induced by intravenous injection of midazolam and fentanyl. We used microSelectron HDR-V3 by Oncentra Brachy (Elekta, Sweden), with iridium-192 (Ir-192) for remote afterloading. CT-based planning was employed for each HDR-ISBT session. Under US guidance, fourteen interstitial needles were inserted into the tumor (Figure 1C). Dose distribution of HDR-ISBT is shown in Figure 1D, and treatment details of HDR-ISBT are summarized in Table 1. Clinical target volume (CTV) was 177 cc, and CTV D90, V100, V150, and V200 values were 13.0%, 75.4%, 32.2%, and 13.5%, respectively. Skin D1cc and D2cc were 8.8 Gy and 8.6 Gy, respectively. Only acute grade 2 dermatitis was observed after HDR-ISBT. The right inguinal lymph node metastasis significantly regressed 5 months after HDR-ISBT (Figure 2A, B). However, new subcutaneous metastasis appeared in the right breast and right popliteal fossa. We administered another HDR-ISBT (30 Gy/2 fractions in 1 day) for each new lesion based on the patient’s prognosis. Details of additional HDR-ISBT for subcutaneous metastasis to the right breast and right popliteal fossa are shown in Figures 3 and 5, respectively. There was no acute toxicity associated with the second HDR-ISBT. The right inguinal lymph node metastasis further shrank 11 months after the initial HDR-ISBT (Figure 2C, D), and the subcutaneous metastasis to the right breast and right popliteal fossa also regressed (Figures 4 and 6). At the time of writing this report, the patient is still alive, and tumor re-growth was not observed at any of the three sites treated with HDR-ISBT during the 41 months of follow-up from the initial HDR-ISBT. Moreover, HDR-ISBT-related late toxicity was not observed during this follow-up period.
Fig. 1
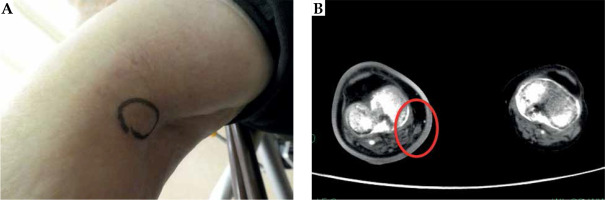
The right inguinal lymph node metastasis of extraskeletal myxoid chondrosarcoma (EMC). A) Pre-treatment gross finding, B) Pre-treatment sagittal CT image, C) Photograph of the needle application of high-dose-rate (HDR) interstitial brachytherapy (ISBT), D) Dose distribution of HDR-ISBT. The light blue dot line is CTV, and the red solid line is 100% (15 Gy) isodose line
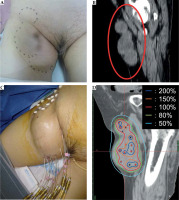
Fig. 2
A) Post-treatment gross finding, B) Post-treatment sagittal CT image of the right inguinal lymph node metastasis at 5 months after high-dose-rate (HDR) interstitial brachytherapy (ISBT), C) Post-treatment gross finding, D) Post-treatment sagittal CT image of the right inguinal lymph node metastasis at 11 months after HDR-ISBT
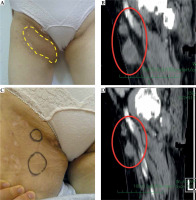
Fig. 3
The right breast metastasis of extraskeletal myxoid chondrosarcoma (EMC). A) Pre-treatment gross finding, B) Pre-treatment axial CT image, C) Photograph of the needle application of high-dose-rate (HDR) interstitial brachytherapy (ISBT), D) Dose distribution of HDR-ISBT. The light blue dot line is CTV, and the red solid line is 100% (15 Gy) isodose line
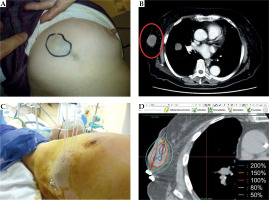
Fig. 4
Six months after second palliative high-dose-rate (HDR) interstitial brachytherapy (ISBT); the right breast metastatic tumor significantly decreased in size. A) Post-treatment gross finding; B) Post-treatment axial CT image
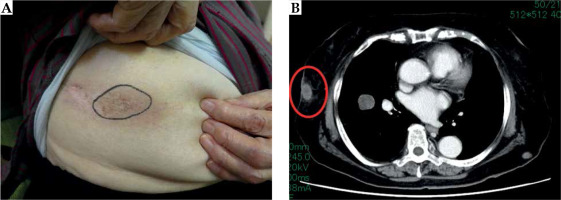
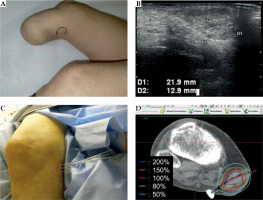
Fig. 5
The right popliteal fossa metastasis of extraskeletal myxoid chondrosarcoma (EMC). A) Pre-treatment gross finding, B) Pre-treatment US image, C) Photograph of the needle application of high-dose-rate (HDR) interstitial brachytherapy (ISBT), D) Dose distribution of HDR-ISBT. The light blue dot line is CTV, and the red solid line is 100% (15 Gy) isodose line
Discussion
We presented a case, in which HDR-ISBT achieved both palliation and excellent local control for metastatic EMC, without an incidence of severe acute and late toxicity. Historically, EMC has been considered an RT-resistant tumor, similar to conventional chondrosarcoma. Due to unavailability of data, the European Society of Medical Oncology and National Comprehensive Cancer Network guidelines recommended that the use of adjuvant or neoadjuvant RT, including BT in STS, also apply to EMC [6, 7]. Based on recent publications, similar to other STS, surgery for localized EMC combined with EBRT showed a better local control compared to surgery alone [8-10].
Extraskeletal myxoid chondrosarcoma can develop distant metastasis, similar to other STS. The most common metastasis site is the lung, although lymph node metastases are seen more often in EMC than in other STS sub-types [15, 16]. Recurrent or metastatic STS is treated using a standard palliative EBRT regimen (for example, 30 Gy/10 frac-tions, 20 Gy/5 fractions, etc.). A previous case series of 17 patients, who received palliative EBRT for sarcoma using 39 Gy in 13 fractions showed durable pain control in 80% of cases, with less toxicity [17]. Tween et al. reported 105 sarcoma patients (137 sites) treated with palliative EBRT using 25 different dose fractionation schedules [18]. A total of 70% of soft tissue and 55% of bone sarcoma patients reported symptomatic improvement. One of the biological characteristics of sarcoma cells is their relatively low (−0.5-5.4) alpha/beta ratio (α/β) [19]. In these previous reports, α/β of 4 was used to calculate a biological effective dose (BED) [17, 18]. Based on these studies, EBRT is widely considered a standard of palliative RT for metastatic sarcoma patients. However, some metastatic sarcoma patients, including the elderly or those with severe pain due to the disease, are not suitable for even 2 or 3 weeks of palliative EBRT. Although 8 Gy in 1 fraction regimen is also used for such fragile patients, it seems insufficient for long-term control of local lesions (BED = 24 Gy, α/β = 4). In contrast, HDR-ISBT with 30 Gy/2 fractions in 1 day, which we used in the presented case, had quite a higher BED (142 Gy, α/β = 4) than EBRT regimens. We consider that the high BED might have enabled long-term local control of the 3 metastatic sites. Brachytherapy can achieve a higher target dose, and yields more conformal dose distribution due to the placement of applicator. Even in palliative setting, especially for patients with slow-growing tumor, large benefits accrue from achieving both symptomatic improvement and long-term local control of a metastatic lesion. Therefore, compared with palliative EBRT, palliative HDR-ISBT is superior in terms of not only short treatment time, but also long-term local control. Under sufficient sedation and analgesia, HDR-ISBT may be performed safely even in older patients.
The optimal radiation dose and fractionation of palliative HDR-ISBT are unknown. In our case, dermatitis was the only HDR-ISBT-related acute toxicity. We focused on the 100% isodose line to prevent overlapping with the skin surface while planning BT to avoid severe skin toxicity. As a result, the skin D1cc and D2cc were under the 100% prescribed dose, and skin toxicity was acceptable in the 3 sites (Table 1). However, further studies are warranted to investigate the optimal radiation dose and fractionation of HDR-ISBT in palliative setting.
Table 1
Treatment details of HDR-ISBT in 3 sites
[i] CTV – clinical target volume, D90 – minimal dose delivered to 90% of target volume, Gr. – grade, HDR-ISBT – high-dose-rate interstitial brachytherapy, Rt – right, Skin Dncc – doses for the most exposed n cc volumes of the skin, Vn – fractional volume of the organ receiving n% of the prescribed dose
Conclusions
We administered HDR-ISBT to a patient with metastatic EMC, and excellent long-term local control was achieved without severe acute and late toxicity. A HDR-ISBT regimen with 30 Gy/2 fractions in 1 day can be a suitable option for both palliation and long-term local control for patients with metastatic EMC.