Purpose
Worldwide, cervical cancer is the fourth most frequently occurring malignancy in women and results in an estimated 530 000 new cases annually with 270 000 deaths [1]. Brachytherapy (BT) as a boost to external beam radiotherapy (EBRT) is the gold standard in the curative management of locally advanced cervical cancer and significantly improves survival [2,3,4]. In the last decades, the development of intensity-modulated radiotherapy (IMRT) has resulted in the reduction of acute and chronic toxicity in pelvic radiotherapy compared to conventional three-dimensional (3D) conformal radiotherapy (CRT), with the same oncological outcome [5,6,7,8,9]. Concerning BT, radiation therapy centres around the world transited from low-dose-rate (LDR) to remote after-loading high-dose-rate (HDR) keeping the same oncological result, as demonstrated in a number of randomized trials [10,11,12,13,14,15,16]. A third type of BT is pulsed-dose-rate (PDR), developed in the 1990s, which combines the radiation safety advantages of after-loading technology and isodose optimization of HDR-BT (applicators and treatment planning system are the same and after-loaders are quite similar) with the theoretical radiobiological advantage of LDR-BT, due to the incomplete repair of the sub-lethal damage between two succeeding pulses [17,18]. From an organizational point of view, PDR-BT is similar to LDR-BT in terms of the dedicated shielded room and nursing care.
Thanks to the use of computed tomography (CT) and magnetic resonance imaging (MRI), treatment planning drastically shifted to 3D optimization, allowing improved tumour coverage control and a decreased dose to organs at risk (OARs). The aim of this retrospective single-centre study was to evaluate results and treatment-related toxicities of IMRT followed by PDR-BT for cervical carcinoma.
Material and methods
Patient characteristics
The inclusion criteria were as follows: 1) patients treated with exclusive IMRT ± chemotherapy followed by PDR-BT boost for primary cervical cancer; 2) confirmation from the multidisciplinary tumour board of the treatment strategy; 3) written informed consent for the use of the patients’ anonymized data for research and educational purposes. Patients’ evaluation included complete medical history and physical examination. Pre-treatment imaging included abdominal and pelvic CT, and/or MRI and/or fluorodeoxyglucose positron emission tomography (FDG PET) scan [19]. For each patient, tumour and treatment characteristics, such as histology, TNM classification and International Federation of Gynecology and Obstetrics (FIGO) clinical stage 2009 [20,21], doses of EBRT, type of chemotherapy and BT implant characteristics, were collected. Patients with urinary bladder or rectal involvement at diagnosis, or relapse requiring salvage treatment, which may have affected morbidity, were not excluded from the analysis, even if suggested by some authors [22,23]. Acute toxicity was defined as toxicities that occurred within six months from the end of BT and it was split into acute genitourinary (GU), gastrointestinal (GI) and haematological toxicity according to the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) scale [24]. Late GU and GI toxicity were defined as toxicities occurring after six months from the end of BT and they were described by the Subjective Objective Management Analytic-Late Effects of Normal Tissues (SOMA-LENT) scale [25].
Treatment characteristics
All patients received whole pelvis image-guided IMRT to a total dose of 45-50.4 Gy (1.8 Gy/fraction, 5 fractions/week), no boost was added to enlarged lymph nodes.
Computed tomography simulation scans were performed acquiring 2.5 mm slices with patients in a supine position. All patients were immobilized during CT simulation and treatment using the dedicated immobilisation device Combifix (CIVCO Medical Solutions, Kalona, Iowa, Unites States of America). CT scans were transferred to the treatment planning system Eclipse (Varian Medical System, Palo Alto, CA).
The pelvic clinical target volume (CTV) included the whole cervix, uterus, parametrial tissue, adnexa (if seen), vagina (length depending on the disease stage: the upper half of the vagina if there was no vaginal involvement or at least 2 cm below known disease), pelvic lymph nodes and any structures partially infiltrated, such as the bladder or rectum wall. Para-aortic nodes were treated if positive or in case of common iliac nodal involvement. Inguinal nodes were included in case of stage IIIA disease. The planning target volume of primary tumour (PTV T) was obtained by expanding the CTV of 1.5 cm in the antero-posterior and latero-lateral directions, and of 1 cm in the cranio-caudal direction. The urinary bladder, rectum (including the anal canal, up to the level of the recto-sigmoid junction), bowels, peritoneal cavity (excluding muscle, bone and great vessels-aorta and inferior vena cava), spinal cord, cauda, and femoral heads were contoured as OARs.
Plan optimization was fulfilled adjusting dose-volume histogram (DVH) points and priorities to best meet OAR dose constraints without compromising the target coverage.
The 95% isodose should encompass at least 97% of the PTV volume and no more than 0.03 cc of the PTV should receive more than 110% of the prescribed dose.
The volume of rectum and bowel receiving 45 Gy (V45Gy) should be less than 60% and 30%, respectively. The volume of bladder receiving 50 Gy (V50Gy) should be less than 35%.
Based on the results of randomised trials [26,27,28,29,30], 45 patients received concomitant platinum-based chemotherapy with or without paclitaxel (5 patients did not receive paclitaxel because of allergy or intolerance).
At the end of IMRT treatment, a gynaecological examination was performed to assess the remaining tumour and any residual pathologic tissue into the parametria or vagina.
Patients with more extended cervical disease at diagnosis were re-evaluated with pelvic MRI.
Endocavitary or hybrid endocavitary-interstitial BT implants were prescribed to 41 (82%) and 9 (18%) patients, respectively, according to patient’s anatomy (e.g. narrow vagina, obliterated fornices, loss of endocervical canal not allowing a tandem placement), tumour size and/or persistent distal involvement of parametria at the time of BT. Interstitial needles were placed under transrectal ultrasound guidance when indicated and feasible, in particular to avoid ureteral stent when present.
A CT scan with the applicator in situ was performed with 2.5 mm slice thickness and 50 ml of diluted iodine into the bladder. In selected cases with interstitial needles close to the rectal wall, 50 ml of diluted contrast was inserted into a tube placed in the rectosigmoid. No patients received intravenous contrast. Images were sent to the planning system, Plato (Elekta-Nucletron) until December 2011 and Oncentra Brachy (Elekta-Nucletron) later.
We did not have the opportunity of performing MRI for dosimetry at the time of BT and we have only used CT scan to delineate target and OARs. Due to the absence of the MRI simulation we could not define any residual gross tumour volume.
The high-risk clinical target volume (HR-CTV) was defined as the whole cervix and any adjacent macroscopic tumour extent at the time of BT, if present [31,32,33,34]. For patients with tumour extension superior to the cervix, the initial infiltration into the uterine corpus was contoured to cover any area potentially at risk [35]. The external wall of the bladder, rectum and bowel loops surrounding the CTV was delineated as OARs.
Manual or graphical optimization was used to improve the dose to CTV, lowering the dose to OARs. According to our internal guidelines, we prescribe the BT dose to the HR-CTV; moreover 90% (D90) of HR-CTV should be covered by at least 100% of the prescribed dose, and 2 cm3 (D2cc) of the bladder and rectum volume should receive less than 80% and 70% of the prescribed dose, respectively. After optimised plan validation, BT started delivering hourly pulses around the clock by a cable-driven iridium-192 (192Ir) source. Total doses from IMRT and BT were converted to equivalent doses at 2 Gy per fraction (EQD2, using the linear quadratic model, with α/β = 10 Gy for tumour and 3 Gy for OARs, and a half-time repair of 1.5 hours) [32]. The dose constraints used in our institute for the combined dose to D2cc of the bladder and rectum were 80-90 Gy EQD23 and 70-75 Gy EQD23, respectively. The total D90 HR-CTV should be higher than 80-85 Gy EQD210.
A pelvic MRI, tumour markers and clinical assessment of adverse events were recorded at 2-3 months and then every 3-4 months for the first 2 years and every six months thereafter. Dilator use was recommended to reduce the risk of vaginal stenosis.
Statistical analysis
Binomial exact confidence intervals (CI) were calculated for the risk of overall and site-specific acute toxicities. The association between clinical and dosimetric variables with acute toxicities was evaluated using the chi-square test. The association between clinical and dosimetric variables and the risk of chronic toxicities was evaluated using Poisson regression, taking into account the length of the period at risk. Rate ratios and 95%CI were reported. Tumour persistence was defined as the incomplete disappearance of the tumour 6 months after the end of BT. The primary endpoint was local control (LC), which was defined as absence of disease in the cervix, upper vagina and parametria. Overall survival (OS) was calculated from the start of IMRT to the time of death from any cause. Progression-free survival (PFS) was calculated from the end of BT to the time of local, nodal and/or distant tumour relapse, tumour progression in patients with persistent disease, or death, whichever occurred first. OS and PFS were calculated using the Kaplan-Meier estimator. The log rank test was used to assess differences of PFS and OS between groups. Patients alive without any event were censored at the time of the last follow-up. A P value < 0.05 was considered statistically significant for all the analyses. All the analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
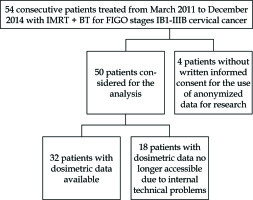
From March 2011 to December 2014, 50 consecutive patients with histologically proven cervical carcinoma FIGO stages IB1-IIIB were treated with PDR-BT after IMRT treatment, with curative intent. Patient, tumour and treatment characteristics are listed in Table 1 and Figure 1. Among 45 patients treated with concomitant chemotherapy, 80% of them showed G ≤ 2 haematological toxicity. No G4 toxicity was reported. No significant difference was found in treatment duration between patients with severe blood toxicities (G > 2) and patients with no severe blood toxicities (G ≤ 2) (p = 0.83).
Table 1
Patient, tumour and treatment characteristics (N = 50 total pts)
Eleven patients with FIGO stage ≥ IIB underwent pelvic MRI at the end of IMRT, in order to evaluate parametrial residual involvement before starting BT. All but one of these eleven patients showed a complete (50%) or partial (40%) radiological response; one patient had stable disease before BT implant.
We did not have the opportunity to perform MRI for dosimetry at the time of BT and we have used only CT scan to delineate the target and the exterior contour of OARs. Due to the absence of MRI simulation we could not define the residual gross tumour volume (GTV).
Dosimetric records were accessible only for 32 patients (Table 2) stored on Oncentra software; the treatment plans of 18 patients calculated with Plato software are no longer accessible due to internal technical problems.
Table 2
Dosimetric data to HR-CTV and OARs. Doses from IMRT and BT were converted to equivalent doses at 2 Gy per fraction (EQD2, using the linear quadratic model, with α/β = 10 Gy for tumour and 3 Gy for OARs)
Acute toxicity data were available for 49 (98%) patients. GU and GI acute toxicity of any grade was observed in 20 (41%, 95% CI: 27-56%) and 12 (24%, 95% CI: 13-39%) patients, respectively. At univariate analysis, no statistically significant acute toxicity increase was observed for any tumour or treatment characteristics, apart from an association between type of BT (endocavitary vs. endocavitary and interstitial) and GI acute toxicity (p = 0.023).
Late toxicity was available for 47 patients. Overall, 4 (8.5%) patients had grade ≥ 2 urinary toxicity, 11 (23.4%) patients experienced grade ≥ 2 rectal toxicity and 18 (38.3%) patients grade ≥ 2 vaginal toxicity. Five patients (10.6%) had grade 4 rectal toxicity requiring colostomy; one of them developed a small-bowel occlusion and fistula mainly associated with suspected disease progression (Table 3). The correlations between GI-GU late toxicity by tumour and treatment characteristics and dosimetric data (available only for 32 patients) are reported in Tables 4 and 5. Regarding the external beam treatment of the 5 patients with high grade late toxicity, 3 patients received IMRT to a total dose of 50 Gy at 2 Gy/fraction (out of 11 patients in the series; 27.7%) and 2 patients received IMRT to a total dose of 45 and 50.4 Gy at 1.8 Gy/fraction (out of 39 patients in the series; 5.1%) (p = 0.031, χ2 test). In reference to BT, 4 patients were treated with a dose rate of 0.6 Gy/h (out of 22 patients in the series; 18.1%) and one patient with a dose rate of 0.5 Gy/h (out of 25 patients in the series; 4%); none of the 3 patients treated with a dose rate of 0.4 Gy/h developed G4 toxicity (p = 0.097, χ2 test for trend). Only one of these 5 patients received salvage treatment (chemotherapy for a pelvic recurrence) between the end of BT and the onset of symptoms of G4 toxicity.
Table 3
Characteristics of patients with G4 late toxicities (≥ 6 months after BT). Total doses from IMRT and BT were converted to equivalent doses at 2 Gy per fractions (EQD2, using the linear quadratic model, with α/β = 10 Gy for tumour and 3 Gy for OARs)
[i] IMRT – intensity-modulated radiation therapy, BT – brachytherapy, IMRT dose and dose/fraction – P – pelvic nodes, P + PA – pelvic + paraaortic nodes, BT type – E – endocavitary, E + I – endocavitary + interstitial, NA – not available, DOD – dead of disease, DOOD – dead of other disease, AWD – alive with disease, NED – no evidence of disease
Table 4
Univariate analyses of correlation between GU and GI acute toxicity and tumour stage, type of BT, and dosimetric parameters (49 pts considered for association with clinical parameters, 32 pts considered for association with dosimetric parameters analysed with the Oncentra software). Total doses from IMRT and BT were converted to equivalent doses at 2 Gy per fraction (EQD2, using the linear quadratic model, with α/β = 10 Gy for tumour and 3 Gy for OARs)
| N at risk | GU acute toxicity | GI acute toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0 (%) | G1 (%) | G2 (%) | G3 (%) | P1 | G0 (%) | G1 (%) | G2 (%) | P1 | ||
| Clinical variables | ||||||||||
| Stage | ||||||||||
| I | 18 | 11 (60) | 5 (28) | 1 (6) | 1 (6) | – | 15 (83) | 2 (11) | 1 (6) | – |
| II | 22 | 13 (59) | 6 (27) | 3 (14) | 0 (0) | 0.90 | 17 (77) | 4 (18) | 1 (5) | 0.65 |
| III/IV | 9 | 5 (56) | 3 (33) | 1 (11) | 0 (0) | 0.84 | 5 (56) | 4 (44) | 0 (0) | 0.19 |
| Type of BT | ||||||||||
| Intracavitary | 40 | 24 (60) | 12 (30) | 3 (7) | 1 (2) | – | 33 (82) | 6 (15) | 1 (3) | – |
| Interstitial | 9 | 5 (56) | 2 (22) | 2 (22) | 0 (0) | 0.64 | 4 (44) | 4 (44) | 1 (12) | 0.023 |
| Dosimetric variables | ||||||||||
| EQD2 rectum (Gy)2 | ||||||||||
| ≤ 65 | 16 | – | – | – | – | 13 (81) | 2 (13) | 1 (6) | – | |
| 65 | 16 | – | – | – | – | – | 8 (50) | 7 (44) | 1 (6) | 0.09 |
| EQD2 rectum (Gy)3 | ||||||||||
| ≤ 70 | 27 | – | – | – | – | 18 (67) | 5 (26) | 2 (7) | – | |
| > 70 | 5 | – | – | – | – | – | 3 (60) | 2 (40) | 0 (0) | 0.88 |
| EQD2 bowel (Gy)2 | ||||||||||
| ≤ 65 | 8 | – | – | – | – | 2 (25) | 5 (63) | 1 (12) | – | |
| > 65 | 24 | – | – | – | – | – | 19 (79) | 4 (17) | 1 (4) | 0.07 |
| EQD2 bowel (Gy)3 | ||||||||||
| ≤ 70 | 16 | – | – | – | – | 7 (44) | 7 (44) | 2 (12) | - | |
| > 70 | 16 | – | – | – | – | – | 14 (87) | 2 (13) | 0 (0) | 0.65 |
| EQD2 bladder (Gy)2 | ||||||||||
| ≤ 65 | 1 | 0 (0) | 0 (0) | 1 (100) | 0 (0) | – | – | – | ||
| > 65 | 31 | 18 (58) | 9 (29) | 3 (10) | 1 (3) | 0.15 | – | – | – | – |
| EQD2 bladder (Gy)3 | ||||||||||
| ≤ 70 | 10 | 5 (50) | 3 (30) | 2 (20) | 0 (0) | – | – | – | ||
| > 70 | 22 | 13 (59) | 6 (27) | 2 (9) | 1 (5) | 0.63 | – | – | – | – |
Table 5
Univariate analyses of correlation between late toxicities (≥ 6 months after BT) observed during follow-up and tumour stage, type of BT, and dosimetric parameters (n = 47 pts considered for association with clinical parameters, n = 32 pts considered for association with dosimetric parameters analysed with the Oncentra software). Total doses from IMRT and BT were converted to equivalent doses at 2 Gy per fractions (EQD2, using the linear quadratic model, with α/β = 10 Gy for tumour and 3 Gy for OARs)
| Variable | Any late toxicity | ||||||
|---|---|---|---|---|---|---|---|
| Pts with late toxicities ≥ 2 | Pts at risk | Person-years | Yearly rate | Rate ratio | 95% CI | P1 | |
| Clinical variables | 26 | 47 | 179 | 0.15 | – | – | – |
| Stage | |||||||
| I | 12 | 19 | 68 | 0.18 | 1.00 | ||
| II | 9 | 20 | 79 | 0.11 | 1.35 | 0.52-3.49 | 0.53 |
| III/IV | 0 | 8 | 32 | 0.00 | 2.44 | 0.88-6.72 | 0.09 |
| Type of BT | |||||||
| Intracavitary | 18 | 38 | 149 | 0.12 | 1.00 | ||
| Interstitial | 3 | 9 | 30 | 0.10 | 1.46 | 0.59-3.65 | 0.41 |
| Dosimetric variables | Pts with late GI toxicities ≥ 2 | ||||||
| EQD2 rectum (Gy)2 | |||||||
| ≤ 65 | 2 | 16 | 55 | 0.04 | 1.00 | ||
| > 65 | 6 | 16 | 54 | 0.11 | 3.02 | 0.61-14.96 | 0.18 |
| EQD2 rectum (Gy)3 | |||||||
| ≤ 70 | 7 | 27 | 94 | 0.07 | 1.00 | ||
| > 70 | 1 | 5 | 15 | 0.07 | 0.88 | 0.11-7.14 | 0.90 |
| EQD2 bowel (Gy)2 | |||||||
| ≤ 65 | 4 | 8 | 31 | 0.13 | 1.00 | ||
| > 65 | 4 | 24 | 78 | 0.05 | 0.40 | 0.10-1.59 | 0.18 |
| EQD2 bowel (Gy)3 | |||||||
| ≤ 70 | 4 | 16 | 62 | 0.06 | 1.00 | ||
| > 70 | 4 | 16 | 47 | 0.08 | 1.32 | 0.33-5.27 | 0.69 |
| Dosimetric variables | Pts with late GU toxicities ≥ 2 | ||||||
| EQD2 bladder (Gy)2 | |||||||
| ≤ 65 | 0 | 1 | 5 | 0.00 | 1.00 | ||
| > 65 | 2 | 31 | 107 | 0.02 | n.e. | n.e. | 1.004 |
| EQD2 bladder (Gy)3 | |||||||
| ≤ 70 | 0 | 9 | 35 | 0.00 | 1.00 | ||
| > 70 | 2 | 23 | 77 | 0.03 | n.e. | n.e. | 0.944 |
At 6 months after the end of BT, 46 patients (92%, 95% CI: 81-97%) were disease-free: two patients had tumour persistence (cervix) while two patients developed distant metastases (peritoneal carcinomatosis and distant lymph nodes). Among these 46 patients, after a median follow-up of 33 months (range, 18-51 months), we observed just one local relapse (cervix) and 6 distant metastases (bone and distant lymph nodes), of which 3 also had regional lymph node relapse. In the node-negative (20 patients) and node-positive (30 patients) groups at diagnosis, one (5%) and 6 patients (20%) experienced nodal failures, respectively. Considering the median total dose of IMRT and BT treatment to CTV (80.9 Gy EQD210), no correlation was found between total dose (if < or > 80.9 Gy EQD210) and local failure (p = 0.925).
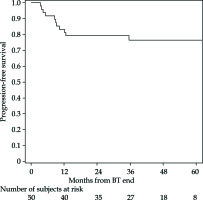
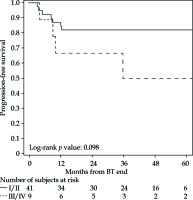
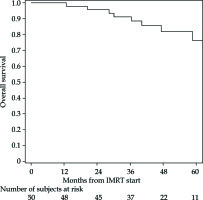
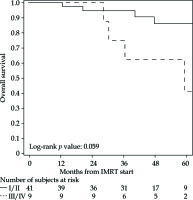
The PFS rates at 1 and 5 years were 83% (95% CI: 69-91%) and 76% (95% CI: 61-86%), respectively (Figure 2 for all stages, Figure 3 for I/II vs. III/IV FIGO stage). Nine cancer-related deaths were registered, and the 3- and 5-year OS rates were 91% (95% CI: 78-97%) and 76% (95% CI: 56-88%), respectively (Figure 4 for all stages, Figure 5 for I/II vs. III/IV FIGO stage).
Discussion
EBRT with concomitant chemotherapy followed by a BT boost is the standard of care for women with locally advanced cervical cancer. IMRT has become widely used in treatment of pelvic cancer due to its dosimetric advantage. In a recent meta-analysis, the reported survival outcomes did not exhibit a significant difference between IMRT and 2D/3D-CRT, though the authors observed a significant benefit with regards to acute toxicity [9]. The development of HDR and PDR remote after loader and new CT-MRI compatible applicators and interstitial titanium or plastic needles paved the way for improvements in BT planning and treatment.
Thanks to the use of CT and MRI, 3D volumetric image-based adaptive BT has been implemented in clinical practice. The systematic analysis of imaging findings before radiation therapy and at the time of BT provides information about tumour extent, topography and regression, mandatory to assess the individual situation and develop an adaptive target approach, according to the GEC-ESTRO recommendations [36,37].
Several trials (with more than 2000 patients) have compared HDR-BT with LDR-BT, obtaining similar results, without significant differences for OS, PFS, or late toxicities even though there is a theoretical better therapeutic ratio in favour of LDR and an increased risk of complications with HDR. Furthermore, HDR allows outpatient treatment with greater patient convenience and safety for treatment personnel. The local failure rate for LDR and HDR-BT ranged from 11% to 24% and from 12% to 27%, respectively [10,11,12,13,14,15,16]. These results were also confirmed by retrospective monocentric or multicentric studies with image-guided adaptive HDR-BT.
Similar control, survival and toxicity rates were obtained with PDR-BT [38,39,40,41,42,43,44,45,46].
In 2008, the GEC-ESTRO group started an international study on MRI-based brachytherapy in cervical cancer (EMBRACE) to standardize MRI-based image-guided adaptive HDR and PDR-BT in a prospective multicenter setting; in 2010, the group initiated a retrospective collection of data on patients treated with the same techniques before starting EMBRACE (retroEMBRACE). In 2016, the EMBRACE-II study was launched to investigate the results obtained with IMRT, concomitant chemotherapy and MRI-based BT, demonstrating excellent local and pelvic control [36,47,48].
Compared to HDR, the lower dose rate of PDR may reduce toxicity by enhancing sublethal damage repair between pulses. A French study compared dosimetric outcomes of PDR and HDR intracavitary BT according to a linear-quadratic model. As recommended, the biological EQD2 were calculated with uniform α/β ratios (10 Gy for tumour and 3 Gy for all OARs) and, only for PDR modelling, with a halftime tissue repair of 1.5 hours for all tissues. Doses to OARs (D2cc of bladder, rectum, sigmoid and small bowel) and to D98 HR-CTV for HDR plans were higher and lower, respectively, in comparison to PDR for equal D90 HR-CTV. The theoretical radiobiological benefit of PDR was observed in particular for patients with significant exposure to OARs (D2cc higher than 20 Gy EQD23). These results suggest that the decrease of the dose per pulse to not exceed the limit of 0.6 Gy/h to the OARs could improve the therapeutic ratio [49].
More recently Schernberg et al. published a study about the prognostic value of GTV shrinkage between diagnosis and the time of BT, evaluated on MRI. A GTV reduction greater than 90% after 3D-EBRT (achieved in 142 out of 247 patients) was independently associated with improved OS, PFS and LC. According to these data, the authors stated that a BT dose de-escalation study could be proposed in well responding patients (with optimal GTV reduction) to decrease the risk of acute and late toxicities without affecting oncological results and, in contrast, a dose escalation (e.g. adding interstitial needles) in poorly responding patients [50].
To the best of our knowledge, our study represents one of the few mono-institutional series of cervical cancer patients treated with IMRT and PDR-BT without surgery.
The 5-year OS and PFS were both 76%, with a crude local control rate of 94% (two patients with residual disease and one with local relapse) considering all FIGO stages. These results in survival and disease control were consistent with those of the most recent literature and are more than promising. Table 6 summarizes the outcomes of published international experiences for PDR-BT with LC and 4-year OS rates ranging from 78.5% to 96% and from 55% to 75%, respectively.
Table 6
Overview of relevant publications on PDR-BT
| Study | Number of patients | FIGO stage | Median follow-up and range (months) | Outcome | Late toxicity > grade 2 |
|---|---|---|---|---|---|
| Rogers et al. [38] | 46 | IB-IVA | 25 (6-55) | 4-year DFS 66% 4-year OS 55% | 6.5% |
| Bachtiary et al. [39] | 57 | IB-IIIB | 50 (5-84) | LC 84.8% 3-year DFS 70% 3-year OS 83% | 7.6% |
| Rath et al. [40] | 48 | IB-IVA | 15 (3-50) | LC 83.4% DFS 80% OS 79% | 6% |
| Charra-Brunaud et al. [41] | 117 | IB-IIIB | 24.3 (5.3-49.5) | 2-year LC 78.5% 2-year DFS 60.3% 2-year OS 74% | 2.6% |
| Lindegaard et al. [46] | 140 | IB-IVA | 36 (6-78) | 3-year LC 91% 3-year OS 79% | 7% |
| Castelnau-Marchand et al. [42] | 225 | IB1-IVA | 38.8 (–) | 3-year LC 86.4% 3-year OS 76.1% | 6.6% |
| Refaat et al. [43] | 40 | IB2-IIB | 30 (7-40) | LC 90% PFS 87.5% OS 100% | 2.5% GI |
| Kumar et al. [44] | 18 | IIB-IIIB | 29 (–) | LC 89% 4-year DFS 71.8% 4-year OS 75% | – |
| Ribeiro et al. [45] | 170 | IB-IVB | 37 (2-136) | LC 96% 5-year OS 65% | 6% GU 5% GI 5% vaginal |
| Schernberg et al. [50] | 247 | IB1-IVB | 50 (6-150) | LC 88% 5-year PFS 66% 5-year OS 71% | – |
| Our study | 50 | IB1-IVB | 32 (18-50) | LC 94% 5-year PFS 76% 5-year OS 76% | 6.3% GU 17% GI 8.6% vaginal |
In univariate analysis, no statistically significant acute toxicity increase was observed for any tumour or treatment characteristics, apart from an association between type of BT (endocavitary vs. endocavitary and interstitial) and GI acute toxicity (p = 0.023) (Table 4).
We reported five patients (10.6%) who experienced grade 4 rectal toxicity requiring surgical intervention.
Similar results have recently been published by Lin et al. [51]. They reported long-term outcomes of 300 patients with cervical cancer who were treated with IMRT (no additional boost to positive nodes) and image-guided HDR-BT. After a median follow-up of 7.2 years (range, 5-12.4 years) the 5- and 10-year regional control was 81% and 79%, and OS was 61% and 57%, respectively. Late bowel/bladder grade ≥ 3 toxicity was reported in 33 patients (11%). Derks and colleagues compared their historical dataset of 2D conventional HDR-BT treatments (35 patients) to 3D MRI HDR-BT with and without the use of interstitial needles (91 patients). Overall 1- and 3-year LC was comparable between these groups, whereas the 3- and 5-year OS showed a trend for an improvement in the 3D cohort. Late grade ≥ 3 toxicity in the 2D-BT and 3D-BT group was 17% and 12%, respectively [52].
The multi-institutional EMBRACE study, including more than 1000 patients treated with 3D-EBRT or IMRT and MRI image-guided HDR or PDR-BT, reported late grade ≥ 3 toxicity for bowel and bladder of 5.9% and 5.3%, respectively [53,54].
Unlike other published data [55,56], we did not find any significant association with tumour stage, type of BT or dosimetric parameters considering both soft and hard constraints for the dose received by 2 cm3 of rectum of 65 Gy and 70 Gy, respectively, as proposed in the European and International study on MRI-guided Brachytherapy in locally Advanced Cervical Cancer (EMBRACE II) study [57]. However, due to the small number of patients with dosimetric data available, it is not possible to exclude an association between dose and risk of acute or late toxicity.
It is well known that the simple DVH addition method adds up the IMRT and BT doses of two different 2 cc volumes of OARs, due to the inter- and intra-fraction organ motion, the presence of the applicator (not present in IMRT image data sets) and the deformation of the nearby structures during its placement. Van Heerden et al.proposed a more accurate evaluation using a deformable image registration to accumulate the absorbed dose distribution from the cone-beam CT of daily image-guided EBRT and BT planning MRI [58]. Since the most exposed part of the integrated plans is in the region of the maximum dose of BT, Fröhlich and colleagues suggested a simple method for adding the biologically effective doses of BT and IMRT in which the most exposed 2 cc from HDR-BT were manually identified and delineated on IMRT CT images [59]. A similar investigation could be the object of future study to better predict toxicity.
We are aware of several limitations of a single-centre retrospective analysis such as the limited number of patients, as well as the fact that 18 out of 50 patients had dosimetric data no longer accessible due to internal technical problems.
Nevertheless, although our cumulative D90 HR-CTV was relatively low (median 80.9 Gy EQD210), the data reported in this cohort show excellent local control and survival rates, comparing very favourably with other published reports [38,39,40,41,42,43,44,45,46,50]. Longer follow-up is needed to confirm these results.
Conclusions
In this study, we analyzed acute and chronic toxicity, OS and disease control in cervical cancer patients treated with IMRT and CT-planned PDR-BT. Despite the small sample size, our data support the use of PDR-BT as a boost after IMRT for the treatment of cervical cancer with promising results especially in terms of survival and LC. In recent literature, the use of advanced techniques has been increasingly recommended to improve the quality of treatments and the therapeutic outcome.