Due to the extensive development in the medical area, short-term outcomes of intensive and critical care patients have been drastically improving over the last decades. The psychological repercussions in intensive care unit (ICU) survivors had already been noticed and reported thoroughly in the past century [1–3]. In 2012, at a conference convened by the Society of Critical Care Medicine, the term post-intensive care syndrome (PICS) was established to describe the impairments in the physical, cognitive, and mental state of patients following their stay in an intensive care unit [4]. Simultaneously, the term post-intensive care syndrome in families (PICS-F) was invented to describe the mental consequences in relatives of the patients [5]. It has been noticed that spouses of patients hospitalised in an ICU have higher risk of developing mental disorders [6]. The reason for this could be the fact that the ICU is possibly the most unfamiliar and intimidating part of the hospital because it requires the most advanced monitoring and involves major medical procedures. The admission to such a ward is usually sudden and unexpected, which causes immense stress for the families. Uncertainty and poor prognosis keep the family members constantly in a precarious position. The relatives usually play the role of caregivers and surrogate decision makers for their spouses, parents, children, or siblings. Additionally, recent years marked by the COVID-19 pandemic showed that lack of contact with their hospitalised loved ones could contribute to their psychological distress [7]. Because of all these reasons, family members of critically ill patients are at high risk of anxiety, depression, post-traumatic stress disorder (PTSD), and other psychiatric disorders [8–10]. Their occurrence may be devastating for the relatives and should not be belittled; in some studies as much as 2/3 of family members experience some kind of mental disorder [11]. There have been multiple interventions made for the families of the critically ill, and some of them have proven to be effective, like the introduction of ICU diaries, the proactive engagements of family members in patients’ care or the promotion of “open” ICUs [12–14]. To know which of those family members require closer attention is crucial, because signs of mental distress could be noticed early and adequately dealt with. Even though risk factors for psychological consequences in the ICU patients seem to be thoroughly discussed in the literature [15], we still lack a complex review of possible risk-factors of such a disorder in their relatives. It is important to determine which aspects can increase the risk of PICS-F, in order to provide the best care for both patients and their families [13]. Therefore, we decided to collect and analyse all the available data regarding this issue in a systematic manner. The primary objective of our analysis was to answer the following question: What are the potential risk factors associated with the development of post-intensive care syndrome in the families of adult patients?
METHODS
The PRISMA 2020 guidelines were implemented for appropriate reporting [16].
Eligibility criteria
We included studies that only focused on relatives of adult (18 years or older) patients who were hospitalised in the ICU in the past (who were either discharged or died during hospitalisation). We primarily focused on risk factors associated with the occurrence of PICS-F. PICS-F was defined as the occurrence of one of the following: anxiety, depression, PTSD, complicated grief, burden/overload, or activity restriction [5–8]. We included studies in which full, peer-reviewed reports were published before the day of the search (23 August 2022). Additionally, the papers had to be published in English language, regardless of the year of publication. Qualitative studies, case reports, case series, systematic reviews, meta-analyses, and papers that assessed mental health of relatives only during ICU hospitalisation were excluded.
Information sources
The search was conducted within PubMed, Embase, clinicaltrials.gov, SCOPUS, and Cochrane Library on 23 August 2022.
Search
For the search in various databases, we implemented the following keywords:
Family: ‘family’, ‘families’, ‘next of kin’, ‘relatives’, ‘spouses’, ‘loved ones’, ‘caregivers’, AND
Post-intensive care syndrome: ‘post-traumatic stress disorder’, ‘depression’, ‘anxiety’, ‘post-intensive care syndrome’, ‘burden’, ‘complicated grief’, AND
Setting: ‘critical care’, ‘intensive care’, ‘critically ill’
Complete search strings are available in the Supplementary Material.
Study selection and data collection process
After importing all the papers from the initial search using the search string, 3 independent investigators (ZP, NR, KMe) assessed studies by analysing titles and abstracts (via Mendeley®). The study was processed further if all adjudicators agreed to include the paper for review. If only 2 reviewers agreed to proceed with the manuscript, a second assessment of the paper was performed by a fourth investigator (ŁK).
Data items
In the description of the studies we included: authors, year of publication, type of study, relatives’ and patients’ characteristics (number of individuals, sex ratio, median or mean age, relationship between patients and relatives, organ failure severity of patients, enrolment criteria), time-point at which mental health assessment of families was performed, mental health assessment tools, any risk factors for the occurrence of PICS-F, and the frequency of PICS-F. We analysed how many times a risk factor appeared in the included papers and how many times a risk factor achieved statistical significance. Multivariable analyses took priority over univariable analyses.
Quality assessment
The Newcastle-Ottawa scale (NOS) was implemented to assess the quality of cohort studies [17]. A modification of NOS was introduced to assess the quality of cross-sectional studies [18]. The total NOS score of each study was converted to the Agency for Healthcare Research and Quality standards [19]. Risk factors derived from random controlled trials were combined with risk factors from good-quality cohort studies. Thus, the studies were rated as either good, fair, or poor. Each of the authors independently participated in the quality assessment of the included studies. Any disagreements were resolved by discussion.
RESULTS
Study selection
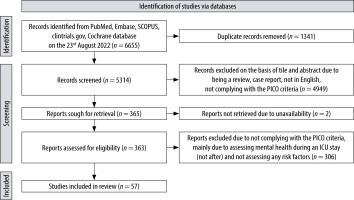
The complete study selection process is depicted in Figure 1. In total, there were 39 prospective cohort studies [20–36, 38, 40, 41, 43–45, 47, 50–52, 54–56, 58–60, 62, 63, 66, 68–70], 6 randomised controlled trials (RCT) [37, 39, 42, 46, 49, 61], and 6 cross-sectional studies [48, 53, 57, 64, 65, 67].
Quality assessment
The Newcastle-Ottawa Scale quality assessment was implemented in 45 studies (the 6 remaining studies were RCTs). Overall, 15 studies were rated as “good” [26–28, 30, 32, 40, 56, 57, 60, 63, 64, 66, 67, 69, 70], 7 studies as “fair” [22–24, 33, 43, 54, 68], and 23 studies as “poor” [20, 21, 25, 29, 31, 34–36, 38, 41, 44, 45, 47, 48, 50–53, 55, 58, 59, 62, 65] (Suppl Table 1). The majority of papers were lacking in terms of the selection criterion: “demonstration that outcome of interest was not present at start of study”, because they did not exclude relatives with mental health disorders such as depression, anxiety, or PTSD. Only 14 studies excluded participants due to the above-mentioned issue [26, 27, 30, 48, 50, 51, 53, 56, 57, 63–67]. A number of studies included only relatives of survivors [25, 33, 41, 43–46, 50, 59, 63, 67–70] or only relatives of deceased patients [23, 37, 42, 48, 55, 62, 65], which limited the representativeness of the exposed cohorts. Out of 45 assessed studies 16 did not implement multivariable analyses and did not control for the most important confounding factors such as gender, age, or marital status. Also, the outcomes were often self-reported by the participants (n = 20) [21, 22, 29, 31, 34–36, 38, 41, 48, 51, 52, 55, 58, 59, 62, 65, 68, 69]. A complete quality assessment is presented in the Supplementary Material.
TABLE 1
Studies included in the review
| Author (year) [Ref.] | Study type | Number of relatives | PICS-F area | Time-point of PICS-F assessment | Statistically significant risk factors for anxiety | Quality |
|---|---|---|---|---|---|---|
| Naef et al. (2021) [20] | PC | 214 | ANX DEP PTSD | Within the 1st month after ICU | MA: death of a patient | POOR 5/9 |
| Meyers et al. (2020) [21] | PC | 103 | ANX | 3 and 6 months after ICU | UA: prior mental health history, relative’s anxiety during ICU, patient’s anxiety | POOR 3/9 |
| Lester et al. (2020) [22] | PC | 96 | ANX | 3 and 6 months after hospitalisation | ANCOVA: anxiety at baseline | FAIR 5/9 |
| Tang et al. (2020) [23] | PC | 278 | ANX DEP | 1, 3, and 6 months after patient’s death | MA: severe anxiety symptoms at 1 month after patient’s death, physician-surrogate prognostic communication | FAIR 6/9 |
| Metzger et al. (2019) [24] | PC | 101 | ANX DEP | 3 months after ICU | MA: unemployment, subsequent depression, witnessing CPR, poor neurological outcome, concomitant mental disorders, use of psychotropic drugs | FAIR 5/9 |
| Lee et al. (2019) [25] | PC | 162 | ANX DEP PTSD | ~ 6 months after ICU | MA: pre-existing mental health disorder during the year prior ICU, recent serious physical illness, female sex of a relative, no health problems before ICU admission | POOR 5/9 |
| Fumis et al. (2019) [26] | PC | 186 | ANX | 1 and 3 months after ICU | MA: atheism, lack of previous ICU experience, higher education, cohabitation with a patient | GOOD 8/9 |
| Oliveira et al. (2018) [27] | PC | 118 | ANX DEP | 1 and 3 months after ICU | MA: female sex of a relative | GOOD 7/9 |
| Beesley et al. (2018) [28] | PC | 99 | ANX | 3 months after ICU | MA: history of anxiety, cortisol awakening response | GOOD 7/9 |
| Petrinec et al. (2017) [29] | PC | 48 | ANX DEP PTSD | 1 week, 1 and 2 months after ICU discharge or death | MA: previous history of psychiatric symptoms, previous history of psychiatric symptoms, avoidant coping mechanism, previous history of psychiatric symptoms, emotion-focused coping mechanism | POOR 4/9 |
| Matt et al. (2017) [30] | PC | 143 | ANX DEP PTSD | 3 months after hospitalisation | MA: female sex of a relative, being a spouse, low quality of life of a patient after ICU, death of a patient | GOOD 9/9 |
| McPeake et al. (2016) [31] | PC | 36 | ANX DEP PTSD CS INS | Between 4 weeks to 3 years after ICU | UA: caregiver strain was associated with depression, poor quality of life of the patient, anxiety was associated with anxiety | POOR 4/9 |
| Hartog et al. (2015) [32] | PC | 84 | ANX DEP PTSD | 3 months after ICU | MA: being a spouse, female sex of a relative, lower satisfaction with communication and care | GOOD 7/9 |
| de Miranda et al. (2011) [33] | PC | 102 | ANX DEP PTSD | 3 months after ICU | MA: large ICU (> 12 beds), depressive symptoms at discharge associated with PTSD | FAIR 6/9 |
| Pillai et al. (2010) [34] | PC | 178 | ANX PTSD | 2 months after ICU discharge or death | UA: lower education levels, trauma admission, greater depression associated with PTSD | POOR 5/9 |
| Anderson et al. (2008) [35] | PC | 50 | ANX DEP PTSD CG | 1 and 6 months after ICU | UA: younger age of a relative | POOR 5/9 |
| Meyers et al. (2020) [36] | PC | 103 | DEP | 3 and 6 months after hospital discharge | UA: no college education, baseline depressive symptoms, patient’s depressive symptoms | POOR 2/9 |
| Kentish-Barnes et al. (2017) [37] | RCT | 208 | DEP PTSD CG | 1 month and 6 months after ICU | MA: being a spouse, female sex of a relative, younger age of a patient, relative living alone | N/A |
| Warren et al. (2016) [38] | PC | 100 | DEP PTSD | 3 months after ICU | UA: traumatic brain injury as a cause of ICU admission | POOR 5/9 |
| Downey et al. (2015) [39] | RCT | 193 | DEP | 3 and 6 months after ICU | Path model: younger age of a patient, depression of a relative during hospitalisation, being a spouse, death of a patient in the ICU | N/A |
| Davydow et al. (2013) [40] | PC | 1212 | DEP | A maximum of 2 years after ICU | MA: female sex of a spouse, disability of patients after hospitalisation | GOOD 8/9 |
| Choi et al. (2013) [41] | PC | 50 | DEP | 2 months after ICU | UA: difficult financial situation, relative who lived with a patient prior to an ICU hospitalisation, unemployment, limited activity of a patient prior to an ICU hospitalisation | POOR 4/9 |
| Gries et al. (2010) [42] | RCT | 226 | DEP PTSD | At least 6 months after ICU | MA: female sex of a relative, education, fewer years of association with a patient, psychotropic drugs taken by relatives prior to the ICU hospitalisation, psychiatric counselling prior to the ICU hospitalisation, neurologic counselling prior to the ICU hospitalisation | N/A |
| Douglas et al. (2010) [43] | PC | 370 | DEP | 2 months after ICU | MA: female sex of a relative, worse condition of a patient during hospitalisation, institutional residency 2 months after discharge | FAIR 6/9 |
| Van Pelt et al. (2010) [44] | PC | 48 | DEP LD | 2, 6 and 12 months after initiation of mechanical ventilation | MA: male sex of a patient, tracheostomy, higher education of a patient, lower patient’s activity post-ICU | POOR 4/9 |
| Van Pelt et al. (2007) [45] | PC | 169 | DEP LD | 2, 6 and 12 months after initiation of mechanical ventilation | MA: older patient, using paid assistance, pre-ICU functional dependency | POOR 5/9 |
| Douglas et al. (2005) [46] | RCT | 290 | DEP CB | 2 months after ICU | MA: depression of a relative during hospitalisation, children as caregivers, institutional residency 2 months after discharge | N/A |
| Im et al. (2004) [47] | PC | 115 | DEP | 2 months after ICU | MA: greater caregiver support | POOR 5/9 |
| Cleiren et al. (2002) [48] | CSS | 95 | DEP PTSD | ~ 6 months after death in the ICU | UA: female sex of a relative, being a spouse or a parent | POOR 4/10 |
| Wendlandt et al. (2018) [49] | RCT | 306 | PTSD | ~ 3 months after initiation of mechanical ventilation | MA: depression of a relative during hospitalisation associated with PTSD, patient’s unresponsiveness | N/A |
| Choi et al. (2018) [50] | PC | 99 | PTSD | 3 and 6 months after ICU | MA: caregiver anxiety during ICU hospitalisation, bond with the patient | POOR 6/9 |
| Schoeman et al. (2017) [51] | PC | 60 | PTSD | 3 months after ICU admission | UA: unemployment of a relative | POOR 7/9 |
| Trevick et al. (2017) [52] | PC | 30 | PTSD CG | 1 and 6 months after enrolment in the ICU | UA: daily visits at the ICU, persistent perceived pain | POOR 5/9 |
| Wintermann et al. (2016) [53] | CSS | 83 | PTSD | ~ 5 months after transfer from ICU to rehabilitation facility | MA: longer ICU hospitalisation, psychiatric disorders in an ICU patient | POOR 7/10 |
| Kentish-Barnes et al. (2015) [54] | PC | 475 | PTSD CG | 6 months after ICU | MA: patient died while intubated, female sex of a relative, relative living alone, no chance to say the final goodbye, presence at the time of patient’s death, patient did not breathe peacefully, not understanding the concept of brain death | FAIR 6/9 |
| Andersen et al. (2015) [55] | PC | 51 | PTSD | 2 months after ICU | UA: higher patient’s APACHE II score, longer ICU LOS, female sex of a relative, lower educational level of a relative, anxiety of a relative at admission of a patient | POOR 4/9 |
| Fumis (2015) [56] | PC | 184 | PTSD | 1 month after ICU | MA: younger age of a patient, death of a patient, anxiety and depression of a relative during an ICU hospitalisation | GOOD 8/9 |
| Zimmerli et al. (2014) [57] | CSS | 101 | PTSD | ~ 2.5 years after cardiac arrest | MA: female sex of the relative, history of the depression, therapeutic measures perceived as insufficient | GOOD 9/10 |
| Sundararajan et al. (2014) [58] | PC | 63 | PTSD | 3 months after ICU | UA: anxiety during ICU hospitalisation was associated with PTSD | POOR 5/9 |
| Dithole et al. (2013) [59] | PC | 28 | PTSD | 6 months after ICU | UA: female sex of a relative | POOR 5/9 |
| Azoulay et al. (2005) [60] | PC | 284 | PTSD | 3 months after ICU | MA: cancer of a patient, higher APACHE II score, death of a patient, children of patients, female sex of a relative, relatives who felt the information from medical team was incomplete, involvement of family members in everyday decisions | GOOD 7/9 |
| Jones et al. (2004) [61] | RCT | 104 | PTSD | 6 months after ICU | UA: anxiety and depression of a relative during an ICU hospitalisation | N/A |
| Kentish-Barnes et al. (2018) [62] | PC | 117 | CG | 9 months after patient’s death | UA: not understanding the concept of brain death | POOR 5/9 |
| Vallet et al. (2019) [63] | PC | 191 | CB | 6 months after ICU | MA: lower daily activity of a patient | GOOD 7/9 |
| Myhren et al. (2010) [64] | CSS | 354 | PD | 1 month after ICU | MA: unemployment status, more environmental strain, less hope for the situation to get better, absence from work, patient still in hospital/institution at the time of evaluation | GOOD 9/10 |
| Siegel et al. (2008) [65] | CSS | 41 | ANX DEP PANIC CG | 3 to 12 months after patient’s death | UA: being a spouse, suffering from an additional stressor, the patient’s sickness duration < 5 years, failure to find the comforting physician | POOR 4/10 |
| Azoulay (2022) [66] | PC | 602 | ANX DEP | 3 months after ICU | MA: patient was a COVID-19 patient, family member is female, younger family member, lower level of social support, death of a patient | GOOD 9/9 |
| Fu et al. (2021) [67] | CSS | 554 | CB | Unclear | MA: younger age of a relative, higher education of a family member, being other than a spouse, higher caregiving time each day, older patient, poor health of a patient, prior chronic disease of patient, worse economic situation, not being covered by a medical aid system | GOOD 8/10 |
| Heesakkers et al. (2022) [68] | PC | 166 | ANX DEP PTSD | 3 and 12 months after ICU | MA: prior mental health disorders in family | FAIR 6/9 |
| Milton et al. (2021) [69] | PC | 62 | CB | 3 months after ICU | MA: worse ICU outcome of a patient | GOOD 7/9 |
| McPeake et al. (2022) [70] | PC | 170 | ANX CS INS | 12 months after ICU | MA: pre-ICU mental health disease in critically ill patient, younger caregiver age | GOOD 7/9 |
[i] ICU – intensive care unit, PC – prospective cohort study, RCT – randomised controlled study, CSS – cross-sectional study, ANX – anxiety, DEP – depression, PTSD – post-traumatic stress disorder, CG – complicated grief, CB – caregiver burden, CS – caregiver strain, LD – lifestyle disruption, INS – insomnia, UA – univariable analysis, MA –multivariable analysis
Post-intensive care syndrome in families
The summary of included studies is presented in Table 1.
Overall, the frequency of PICS-F varied from 2.5 to 69%. The areas of PICS-F that seemed to be the most frequent were complicated grief (median = 46%; IQR = 27–49.9%) and caregiver burden/strain (the median = 37%; IQR = 22.7–62.7%). The median frequencies of anxiety, depression, and PTSD were similar: 31.3%, 24.7%, and 30.5%, respectively (Supplementary Material).
The studies varied in terms of PICS-F assessment: PTSD was the most frequently studied outcome (n = 27) [20, 25, 29–35, 37, 38, 42, 48–61, 68], along with depression (n = 27) [20, 23–25, 27, 29–33, 35–48, 65, 66, 68], anxiety (n = 20) [20–35, 65, 66, 68, 70], caregiver burden/strain (n = 11) [31, 35, 37, 46, 52, 54, 62, 63, 67, 69, 70], lifestyle disruption (n = 3) [44, 45, 64], insomnia (n = 2) [31, 70], complicated grief (n = 1) [65], and panic (n = 1) [65].
The methods used to assess the psychological status in the studies varied, with the most commonly applied being the following: Impact of Event Scale – Revised (IES-R) (n = 12) [31, 33, 34, 37, 49, 51, 52, 54, 57, 58] and Impact of Event Scale (IES) (n = 7) [27, 30, 32, 35, 56, 60, 61] for PTSD, Hospital Anxiety and Depression Scale-Depression (HADS-D) for depression (n = 17) [20, 23, 24, 26, 27, 29, 30–33, 35–37, 66, 70], Hospital Anxiety and Depression Scale-Anxiety (HADS-A) for anxiety (n = 17) [20–24, 26–35, 66, 70], and Inventory of Complicated Grief (ICG) for complicated grief (n = 4) [35, 37, 54, 62] (Supplementary Material). In addition, the studies used slightly different cut-off scores within the same assessment tools, while in several studies it was not specified. The studies also varied in terms of the time-point at which they assessed the outcomes. Three months was the most frequent time-point of PICS-F assessment (n = 33) [21, 23, 24, 26–28, 30, 32, 33, 36, 38, 49–51, 58, 60, 66, 68, 69], followed by 6 months (n = 26) [21, 23, 30, 32, 33, 35–38, 42, 44, 45, 49–51, 55, 58, 60, 63], one month (n = 14) [23, 27, 28, 30, 37, 39, 54, 59], and 2 months (n = 11) [30, 36, 43, 45–49, 58].
Risk factors for PICS-F
In total, 51 potential risk factors for PICS-F were reported in the literature. Three different categories of risk factors could be distinguished. For example, any variables describing the patients were assigned to the patient-related risk factors. The same was done in regard to the relatives. Importantly, factors presumed to be related to medical personnel were grouped under medical-staff-related risk factors.
The most common were relative-related risk factors (n = 27) [26–30, 32, 35, 37, 39, 40, 42, 44, 50, 52, 54, 56, 57, 60, 62, 64, 66–68, 70], followed by patient-related (n = 16) [25, 30, 37, 39, 40, 44–46, 49, 54–56, 60, 61, 63–67, 69, 70] and medical-staff-related (n = 8) factors [23, 24, 32, 33, 54, 57, 60, 65] (Table 1).
The risk factors that emerged from “good”-quality studies are collectively presented in Table 2. Hence, we distinguished 9 patient-related risk factors, 22 relative-related risk factors, and 2 medical-staff related risk factors. In terms of patient-related risk factors, there was a trend showing the relationship between a patient’s worse condition, their lower daily activity, death, and the higher occurrence of PICS-F. When it comes to the relative-related risk factors, social, economic, and psychiatric factors played a significant role: PICS-F especially emerged in females, spouses, in relatives with worse economic situations, and in relatives with a history of mental disorders. Lastly, medical-staff risk factors were associated with failed communication between relatives and the medical staff, and therapeutic measures perceived as insufficient.
TABLE 2
Summary of “good”-quality risk factors associated with the development of PICS-F
| Patient-related | Death [30, 39, 56, 60, 66] Worse condition during ICU stay [49, 60, 67, 69] Younger age [37, 39, 56], older age (for caregiver burden) [67] Lower level of activity before or after ICU stay [30, 40, 63] Residence in an institution after ICU stay [46, 64] Prior chronic disease [67] Mental disorders before or after ICU stay [61, 70] Patient suffered from COVID-19 [66] |
| Relative-related | Female sex [27, 30, 32, 37, 40, 42, 57, 60, 66] Being a spouse [30, 32, 37, 39] Mental disorders during patient’s ICU stay [39, 46, 49, 56, 61] History of mental disorders [28, 42, 57] Younger age [66, 67, 70] Worse economic situation [57, 64, 67] Being a child [46, 60, 67] More hours spent daily helping a patient [67] Presence of additional stressors [30, 64] Being a parent [67] Higher level of education [26, 67] Lower level of education [42] Atheism [26] Not being covered by a medical aid system [67] Lower level of social support [66] Living alone [37] Living with the patient [26] Fewer years of association with patient [42] Being a surrogate decision-maker [60] Less hope for the situation to get better [64] Lack of previous ICU experience [26] Cortisol awakening response [28] |
| Medical staff-related | Lower satisfaction with communication and care [32, 60] Therapeutic measures perceived as insufficient [57] |
DISCUSSION
In this systematic review, we focused on identifying risk factors associated with the development of PICS-F. We described over 50 potential risk factors of which 33 came from the studies of good quality or random-controlled trials. Importantly, most of the included studies presented poor quality.
Risk factors
By synthesizing the included studies, we were able to distinguish 3 main types of risk factors associated with PICS-F: patient-, relative-, and medical-staff-related.
In terms of patient-related risk factors, the ones that seem to account for the development of PICS-F are the risk factors oriented around the severity of the disease and its negative consequences. It is well documented that physical and mental disability are widely spread in survivors of critical illnesses [71]. This explains why 2 studies reported “residence in an institution after ICU stay” as a risk factor for PICS-F. Additionally, because family members often become caregivers of those patients, they are at higher risk of experiencing caregiver stress, which itself is a part of PICS-F and is associated with depression and other mental health disorders [72]. The younger age of a patient worsens the family outcome as well. Both critical illness and death of younger patients are associated with greater stress and with failure to reconcile with unfavourable outcomes [73]. These risk factors can be supplemented with an additional finding: no comorbidities before ICU stay, which is probably related to younger age, sudden critical illness, and family shock. Of note, one study reported older age as a risk factor for caregiver burden [67], whereas other studies reported younger age as a risk factor for depression and PTSD [37, 39, 56]. Importantly, death of a patient was not always identified as a significant risk factor for PICS-F (reported in 6 out of 17 studies that explored “death” as a variable related to the PICS-F). This finding is in line with the above-mentioned considerations, i.e. that a fraction of patients who survive the ICU may require excessive care and their quality of life may be significantly reduced. This would result in relatives experiencing higher burden, higher stress, and lifestyle disturbances that promote the occurrence of PICS-F in a similar fashion as would the death of a patient.
When it comes to relative-related risk factors, female sex is one of the most well-documented among the included literature [27, 30, 32, 37, 40, 42, 57, 60, 66]. This association is often reported regarding depression, anxiety, and PTSD, which are all part of the PICS-F spectrum [74–76]. Being a spouse is another often cited variable, because mental health disorders are more prone to appear in spouses of patients than in other relatives. This may be partially explained by the fact that spouses often become caregivers of the ICU survivors, which is associated with great burden [77–79]. This finding can be additionally strengthened by the other risk factor, i.e. a worse economic situation [80]. Several studies also documented the significant role of a relative’s medical history of mental health disorders in increasing the frequency of PICS-F occurrence. An experience of the ICU hospitalisation of a loved one is associated with a very stressful event and may stand as a risk factor for the recurrence of depression and other mental health disorders [81].
Medical staff-associated risk factors are mostly interdependent and refer to a poor relationship between medical staff and the families of ICU patients. For instance, feeling that information from the medical team is incomplete may be connected with lower satisfaction with communication and care and also with therapeutic measures perceived as insufficient (especially in patients with poor prognosis and withdrawal of life support). Interestingly, one of the relative-related risk factors was not understanding the concept of brain death, which is probably closely related to the lack of successful communication. Additionally, a failure to find a supportive healthcare provider, when experiencing high stress, could strengthen the dissatisfaction of the hospitalisation process and account for the PICS-F phenomenon.
Limitations
Most studies were conducted in Western countries (n = 43). Of note, there is a considerable difference in terms of healthcare, income, culture, and structure of societies between these countries and other parts of the world. This divergence could influence the way the risk factors shape the development of PICS-F. For instance, because developed countries may exhibit higher ICU survival rates, the importance of certain outcomes, e.g. caregiver burden, may be markedly different for countries with higher mortality rates, where, in contrast, complicated grief may be expressed more strongly.
The heterogeneity among the studies was noticeable in terms of study type, selection of the participants, representativeness of patient populations, mental health screening-tools, and assessment of the outcomes (outcome defined as either continuous change in psychiatric scores or as the presence of clinically significant PICS-F). Most of the involved studies focused on 3 PICS-F areas: PTSD (27 studies), depression (27 studies), and anxiety (18 studies), whereas other components of PICS-F were less frequently studied. Consequently, PICS-F-related risk factors are significantly determined by these 2 large groups of studies and should be applied cautiously in relation to other PICS-F areas because the risk factors may vary for particular outcomes.
The analysed studies not only used various assessment tools, but also sometimes applied different cut-off scores for depression, anxiety, and PTSD occurrence. These may introduce a limitation in terms of either frequency of PICS-F or the association between certain risk factors and PICS-F. For example, in 4/17 anxiety studies that implemented the HADS-A tool, anxiety was recognised at a threshold of 11 points (“abnormal”) [24, 27, 29, 34], while the remaining studies introduced a lower threshold of 8 points (“borderline abnormal”).
Most of the outcomes were reported by the participants and not diagnosed by specialists in psychiatry and psychology; however, this is not necessarily associated with less valid observations. Many methods of self-assessment are standardised and designed on the basis of diagnostic criteria of a given disorder. Importantly, patients are experts on their own feelings, emotions, and suffering.
The results of this review are largely shaped by the poor-quality studies and the fact that most of them were not designed to assess the risk factors specifically. However, it must be taken into account that certain studies introduced heterogeneity because they did not exclude participants with present existing mental health disorders (in NOS: “demonstration that the outcome of interest was not present at the start of study”). Such a decision confounds the effect of ICU hospitalisation on the occurrence of PICS-F; however, prior mental health disorders can stand as a risk factor for PICS-F as well. Additionally, several studies did not implement multivariable analyses to determine the significance of risk factors. The analysis of risk factors in this systematic review was based on the quality of the studies and the number of papers that identified each variable as a risk factor for PICS-F; how-ever, the strength of particular factors remains unverified. Lastly, we observed a considerable proportion of studies with loss to follow-up exceeding 20%, which also introduces bias to our analysis.
Future research
This paper identified risk factors that could be used in designing future studies on PICS-F. Additionally, medical staff-related risk factors seem to be the least explored ones (reported in only 9 out of 51 studies). This may come from the fact that these risk factors originated mostly from subjective feelings of relatives and are not easily classifiable. For example, factors such as “lower satisfaction with communication and care” are not only more difficult to assess than, e.g., age of patients, but also require additional resources (interviewers need to contact families after hospitalisation). We believe that there is a need to further explore medical staff-associated risk factors due to the fact that these factors may be one of the few that are controllable and modifiable. Correct identification of such factors could lay the groundwork for proper soft skills education for medical professionals. Healthcare provider-dependent interventions, such as improvements in communication, would potentially help prevent adverse reactions among family members. Further exploration of the influence of lack of proper communication is warranted.
The other reported risk factors (patient- and relative-related) are independent of ICU staff. Therefore, a comprehensive identification of such factors in the ICU setting may not be possible. Nevertheless, modern intensive care with its multidirectionality goes beyond mere hospitalisation and is also oriented to long-term outcomes, primarily of patients, but also of their families. Proper identification of at least some PICS-F risk factors (not necessarily at the level of hospitalisation, but already beyond it) can contribute to planning a multi-stage process of support for the family during the slow process of the patient’s recovery (from rehabilitation, through psychological support, to financial support). Characterisation of at-risk groups can also contribute to the design of future studies focused on interventions in families of ICU patients.
In terms of the studied outcome, as most of the included papers focused on PTSD and depression, more research is needed to evaluate the remaining PICS-F areas.
It is important to remember that this paper focused only on relatives’ mental health that was assessed after hospitalisation. However, there is still a large body of work that focuses on relatives suffering from psychiatric disorders during ICU hospitalisation. Aggregation of this data could further strengthen the evidence of the effect of critical illnesses on families.
CONCLUSIONS
PICS-F is a multifactorial phenomenon that can be aggravated by a considerable number of patient-, relative-, and medical staff-related risk factors. Special attention should be paid to relatives of younger patients with worse prognosis and with the following relative-related risk factors: female sex, being a spouse, and history of mental health disorders. Finally, the medical staff play a role in preventing PICS-F development, not only by maintenance of proper communication, but also by early identification of relatives prone to developing PICS-F.