Introduction
Non-segmental vitiligo (NSV) is a skin pigmentation disorder affecting the functional melanocytes in the epidermis. The prevalence of the disease reported in India was 2.5% [1]. The exact pathogenesis of the disease process in NSV has not been understood yet. One of the important mechanisms for melanocyte death in NSV is its autoimmune basis [2]. Cytokine-mediated interaction of melanocytes with T lymphocytes lead to melanocyte stress and hence, melanocyte death [3]. Loss of functional melanocytes with cytotoxic and helper-T cell infiltration in dermis and epidermis of the perilesional vitiligo skin has been demonstrated in NSV. Available data documented an imbalance in the T-helper cell subsets in the peripheral circulation of patients with NSV [4–7].
Interferon-γ (IFN-γ), a pro-inflammatory cytokine, affects the melanocyte activity and causes apoptosis [8]. Studies have shown that IFN-γ is elevated in NSV compared to controls and expression of IFN-γ is predominant in vitiligo lesion and peripheral blood mononuclear lymphocyte cells (PBMCs) [9, 10]. These data suggest the predominance of a subset of T helper cells which are positive for CD4+IFN-γ+ cells in NSV. IFN-γ does play a role in pathology of several other autoimmune diseases such as systemic lupus erythematosus [11], rheumatoid arthritis and NSV [12]. Interleukin 4 (IL-4), an anti-inflammatory Th2 cytokine, mediates suppression of macrophage functions such as IL-1 and tumor necrosis factor α (TNF-α) production [13, 14]. IL-4 mediates the expansion of Th2 cells from naive cells and acts as an autocrine regulator for differentiated Th2 cells [15]. It is a mediator of the inflammatory process, allergic reactions and autoimmune pathogenesis. The role of T-helper cell cytokines in pigmentation has been reported. IL-4 is shown to directly inhibit melanin synthesis through the JAK2-STAT6 pathway. In addition to the above, IFN-γ and IL-17-dependent IL-6 are also found to interfere in melanin production [16].
An additional distinct subset of the CD4+T-cell lineage, widely accepted as Th17 cells have been found to play a role in the pathogenesis of numerous immune-mediated diseases like psoriasis and rheumatic arthritis [17–19]. The participation of IL-17 cytokine in dysregulation of melanocyte activity in NSV has been described recently [20]. The inhibitory effect of IL-17 on melanocyte activity in vitiligo skin has also been testified [21]. Basak et al. studied the circulatory cytokines of T cells in vitiligo and they found that serum interleukin, IL-17, was correlated with disease severity while there was no difference in serum IL-6, IL-10, TNF-β [22]. The above finding suggests a predominant role of the Th17 cell cytokine in NSV. Few studies have demonstrated decreased levels of transforming growth factor β (TGF-β), the important Treg cytokine, in vitiligo compared to controls [22, 23]. Recent reports have shown the evidence that there is a defect and numbers of Treg cells in vitiligo patients [24, 25]. As Treg cells play an important role in the pathogenesis of several autoimmune diseases, evaluation of Th17/Treg balance in vitiligo is crucial.
Thus, change in the percentage of T helper cells and Treg cells and their function with disease progression may substantiate vitiligo pathogenesis. Several reports have suggested the involvement of T cells in disease pathogenesis and use of cytokine therapy in vitiligo management [26]. Nevertheless, few studies have explored the immunophenotype of T cell subtypes in peripheral circulation in vitiligo [25, 27].
Aim
Henceforth, we planned to evaluate the peripheral T-helper/Treg cell subsets in patients and controls and to correlate with disease activity of NSV patients.
Material and methods
Study subjects
In this cross-sectional study, 80 patients with NSV aged 18–60 years, who were diagnosed with NSV according to the standard guidelines [28] were recruited at the out-patient department of Dermatology & STD of a tertiary care hospital in south India. The patients had not been on any treatment for vitiligo for at least one month prior to enrolment. Patients with other inflammatory skin diseases, pregnancy, infectious diseases/malignancies were excluded. Disease activity was determined by vitiligo index of disease activity (VIDA) scoring of 0–4 [29]. The extent of pigmentation was done by Lund and Browder (LB) score [30]. The control group consisted of eighty age- and gender-matched apparently healthy subjects without any skin and infectious diseases and without a family history of autoimmune diseases. The Institutional Ethics Committee (Human Studies) approved the project (approved as Project number JIP/IEC/2013/5/195). All the study participants were explained in detail about the procedure and written informed consent was obtained. The ethical guidelines as per the World Medical Association (WMA) Declaration of Helsinki ethical principles for medical research for human subjects were abided by while conducting this research [31].
Flow cytometric analysis
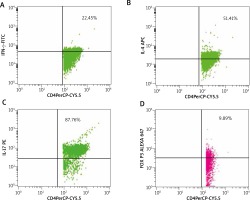
Five millilitres of whole blood was obtained from patients and controls under aseptic conditions. Using standard density gradient centrifugation (Ficoll-Paque), PBMCs were secluded. Further PBMCs were suspended into PBS for further treatment with a mixture of fluorescent antibodies from the Human T-helper/Treg phenotyping kit as per the manufacturer’s instructions (BD Biosciences, California, USA). Then, the PBMCs were analysed using the cytometer (FACS Calibur, Becton Dickinson, California, USA). Detection of the percentage of Th1, Th2, Th17, and Treg subsets was executed by immunofluorescence analyses using the antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), allophycocyanin (APC) and Alexa Fluor® 647. Minimum of 30,000 lymphocytes were obtained for each antibody assortment. Th1, Th2, Th17, and Treg subsets were demarcated by the positivity of CD4 cells with IFN-γ, IL-4, IL-17A, and FoxP3, respectively. CD4 cells of PBMCs were gated primarily, and then the dot plot analyses of subsets were derived from these gated CD4+ cell populations and reported as percentage using Cellquest Pro software (Figure 1).
Figure 1
Analysis of PBMC cells of patients with NSV by flow cytometry. The figure shows the percentages of T cell subsets. A – CD4+ IFN-γ+ showing positive for PerCP-CYP.5 and FITC (22.45%), B – Th2 cells with CD4+IL-4+ positive for PerCP-CYP.5 and APC (51.41%), C – Th17 cells with CD4+IL-17A+ showing positive for Per- CP-CYP.5 and PE (87.86%), D – Treg cells with CD4+FoxP3+ showing positive for PerCP-CY5.5 and Alexa 647 (9.89%)
FITC – fluorescein isothiocyanate, PE – phycoerythrin, PerCP – peridinin chlorophyll protein, APC – allophycocyanin.
Statistical analysis
Statistical analysis of data was done using GraphPad Prism version 5.00 (San Diego, California, USA). Demographic characteristics of the study subjects were evaluated by descriptive statistics. Kolmogorov-Smirnov test was employed to assess the normality of continuous data (data related to variables such as age of onset of disease, duration of disease symptoms, T-cell counts). Depending on the data, parametric data were defined by mean ± standard deviation, and non-parametric data were defined by median and inter-quartile range (IQR). Mann-Whitney U-test was used to estimate the differences in PBMCs with T-helper and Treg cells between the groups. Pearson or Spearman correlation, as appropriate, was used to study the association of the cell counts with the disease activity in vitiligo patients.
Results
The demographic characteristics of participants are given in Table 1. No significant difference in the mean age, body mass index (BMI) between the patients and controls was observed. Mean duration of the disease was 53.95 ±82.84 months, mean age of onset of disease was 35.46 ±15.63 years and mean VIDA score was 2.13 ±1.33.
Table 1
Demographic characteristic of the study participants
Figures 1 A–D shows the analysis of PBMC cells for the T cell subsets. Based on flow cytometry analysis results (Figures 2 A–D), we noted a substantial increase in the proportion of Th1 cells with CD4+IFN-γ+ with median (IQR) of 10.16 (7.39–12.36) in patients as compared to healthy controls 5.23 (3.69–8.12) (p < 0.0001). We observed an increased percentage of Th17 cells positive for CD4+IL-17A+ with median (IQR) 36.41 (25.50–91.45) in patients as compared to controls 34.58 (15.16–62.73) (p = 0.01).
Figure 2
A – Distribution of Th1 cells with CD4+IFN-γ+ (median (IQR: 5.235 (3.69–8.12) vs. 10.16 (7.39–12.36) for patients and controls, respectively), B – Th2 cells with CD4+IL-4+ 25.20 (14.62–51.07) vs. 23.12 (15.39–30.85), C – Th17 cells with CD4+IL-17A+ 36.41 (25.50–91.45) vs. 34.58 (15.16–62.73), D – Treg cells with CD4+FoxP3+ 8.65 (6.83–9.40) vs. 8.13 (6.29–9.43). Analysis was done by Mann-Whitney U test
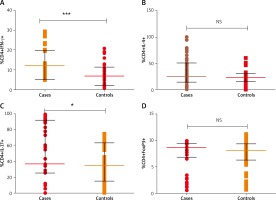
No significant difference was observed in Th2 cells with CD4+IL-4+ between patients and controls (median (IQR) 25.20 (14.62–51.07) vs. 23.12 (15.39–30.85)) respectively (p = 0.06). In case of Treg cells, percentage of cells with stain positive for CD4+FoxP3+ were not significantly different among patients and controls 8.65 (6.83–9.40) and 8.13 (6.29–9.43), respectively, (p = 0.70). Disease activity (VIDA) was not correlated with the percentages of cells (p > 0.05). When doing the subgroup analyses of patients, there was no significant difference in the percentage of Th1, Th2, Th17, Treg cells in active and stable vitiligo patients (p > 0.05).
Discussion
Non-segmental vitiligo is an autoimmune depigmentation disorder involving the skin. Cytokines and T cells play a key role in the disease pathogenesis. Very few studies have investigated the relative immunophenotype of T helper cells and Treg cells in NSV. We observed an immune dysregulation with an increase in the percentage of pro-inflammatory Th1 cells with CD4+IFN-γ+ and Th17 cells with CD4+IL-17A+, whilst we did not find any alteration in the anti-inflammatory T cell subsets like Th2 cells with CD4+IL-4+, and Treg cells with CD4+FoxP3+ in NSV, as compared to controls.
Yang et al. [8] had reported a surge in relative percentage of PBMC cells with CD4+IFN-γ+ in lesional vitiligo skin. They postulated that CD8+ cells could be a source of increased IFN-γ and cause melanocyte destruction in the lesional skin. Our results with an increased percentage of peripheral Th1 cells with CD4+IFN-γ+ are in agreement with their results. Contrary to our results, a previous study by Zhou et al. has demonstrated that there was no alteration in CD4+IFN-γ+ cells between patients and controls (p > 0.05) [32].
In the previous reports, elevated IL-17 has been reported to correlate with the extent of depigmentation [22, 33]. Wang et al. have reported that Th17 cells are abundant in the leading edge of vitiligo skin biopsies [34] and further confirmed the association of Th17 cells with vitiligo pathogenesis. Our finding of the increased percentage of peripheral Th17 cells with CD4+IL-17A+ is concordant with their findings. The melanocyte function deteriorates with the imbalance in the cytokine network locally and that circulating IL-17 acts in the skin by triggering the production of cytokines from dermal fibroblasts and keratinocytes [21]. This could probably explain the elevation of Th17 cell subtype in circulation in NSV in the present research.
Increased levels of IL-4 have been reported in previous studies [35, 36]. Our findings show that there was no difference in the Th2 cells with CD4+IL4+ cells between patients and controls. Similar findings obtained by Zhen et al. with no change in Th2 cells with CD4+IL-4+ in patients and controls clarified that the elevation of Th1 and Th17 cell subtypes could probably be due to interplay between the T cell subsets, showing the Th1 and Th17 dominance over the Th2 cells in NSV. They had reported elevated Th1/Th17 cells in peripheral circulation of vitiligo and they had confirmed the mRNA expression of corresponding transcription factors [27] and our results are in line with these results reported by Zhen et al.
In several other autoimmune diseases like psoriasis and SLE, imbalance in Treg cells with Foxp3+ contributes to exhibit their pathogenesis [37]. We found no significant change in peripheral Treg cells positive for CD4+Foxp3+. Zhou et al. reported that there was no alteration in the percentage of Treg cells in PBMCs between NSV patients and healthy controls with comparable CD4+Foxp3+ Treg cells (patients, 5.68 ±2.87% vs. controls, 5.87 ±3.97%; p > 0.05) [32], which is in concordance with our findings. A decrease in expression of Foxp3 in lesional skin compared to normal skin of vitiligo patients had been reported by Ben Ahmed et al. [25]. Klarquist et al. demonstrated that the percentage of CD127lowCD4+CD25+FOXP3+ among peripheral lymphocytes was similar in vitiligo patients and healthy controls [24]. They suggested that Treg cells abstain from migrating to the skin in vitiligo patients because of a decreased expression of skin homing receptors. Zhen et al. had noted no significant difference between cells of Tregs with CD4+ Foxp3+ in active vitiligo, which is similar to our findings which revealed a comparable percentage of peripheral Tregs between patients and controls. In conclusion, we found immune dysregulation in peripheral circulation of patients with NSV, associated with preponderance of Th1 and Th17 subsets.
However, our study had a few limitations. A larger sample size and a replication cohort would have validated our preliminary results further. Expression studies of the T-helper/Treg cytokine genes and their genotype-phenotype correlation would have provided a deeper understanding of the vitiligo pathogenesis.








