Summary
In the field of TAVI, technical advances in device manufacturing are crucial in order to achieve better acute and long-term results. The FlexNav delivery system is characterized by a hydrophilic, integrated sheath with a reduced entry profile diameter; it also features a stability layer aimed at minimizing system manipulations and allowing for precise valve positioning. We report the performance of the FlexNav in patients with severe aortic stenosis undergoing Portico valve implantation. In-hospital outcomes were self-adjudicated according to the Valve Academic Research Consortium-3 definition. Our data suggest that the FlexNav DS, thanks to lower profile and enhanced stability during valve deployment, has the potential to allow for better procedural and clinical results of transcatheter aortic valve replacement with the Portico valve as compared to the 1st Gen-DS.
Introduction
The Portico valve is a self-expanding, repositionable biological valve suitable for transcatheter treatment of aortic valve stenosis. In a randomized comparison with commercially available devices, it was found to be non-inferior for the composite end-point of death/disabling stroke at 1 year in patients at high and extreme surgical risk with severe symptomatic aortic stenosis [1]. In this trial the valve was delivered with the first-generation Portico delivery system, which has an 18F outer diameter for the smaller valves (23 and 25 mm) and a 19F outer diameter for the larger valves (27 and 29). Recently, a new version of the delivery system, the FlexNav, has been introduced. This new delivery system is characterized by a hydrophilic, integrated sheath with a reduced entry profile diameter (14–15 F); it also features a stability layer aimed at minimizing system manipulations and allowing for precise valve positioning [2]. Such technical advances may theoretically improve procedural and clinical outcomes of the Portico valve through reduction of vascular complications and better control of the implantation depth which, in turn, may be associated with less paravalvular leak and permanent pacemaker implantation. Indeed, an excellent safety profile of the FlexNav delivery system has been observed in 2 recently published registries [3, 4].
Aim
In this study we aimed to compare the FlexNav delivery system (FlexNav DS) with the first-generation delivery system (1st gen DS) in terms of in-hospital procedural outcomes of transfemoral transcatheter aortic valve replacement (TAVR) with the Portico valve in a single-center cohort of patients.
Material and methods
We retrospectively compared consecutive patients undergoing TAVR with the Portico valve (Abbott Cardiovascular, Plymouth, MN) and FlexNav DS (FlexNav DS group) with an historical cohort of patients receiving the same valve with the first-generation DS (1st gen DS group) in 2019 and first quarter of 2020, when structural interventions in our institution were stopped because of the COVID-19 sanitary crisis. The FlexNav DS was approved for clinical use in Europe in March 2020. We started using this DS in May 2020 and, for the purpose of this study, we included consecutive patients until July 2021, when the Navitor valve (Abbott Cardiovascular, Plymouth, MN) almost replaced the Portico valve at our institution. Consequently, in order to avoid bias in the comparison between the FlexNav DS and 1st gen DS, patients receiving the Navitor valve were not considered for the purpose of this study. Clinical and procedural data were obtained by revision of hospital patient files and catheterization laboratory registry. In our institution all patients are scheduled for TAVR after a complete diagnostic workup which includes coronary angiography, computed tomography (CT) scan and multidisciplinary Heart Team evaluation. Moreover, after obtaining informed consent, patients are prospectively enrolled in a multicenter registry approved by the Institutional Review Board. TAVR was performed in all patients under sedation by percutaneous transfemoral access with fluoroscopy-guided puncture of the common femoral artery and double Perclose Proglide (Abbott Cardiovascular, Plymouth, MN) preimplantation technique [5].
In-hospital adverse events were defined according to Valve Academic Research Consortium (VARC)-3 criteria and included all-cause death, stroke, bleeding, acute kidney injury and major vascular complications [6]. Data on the incidence of paravalvular leak, new permanent pacemaker implantation and new left bundle branch block were also collected.
Statistical analysis
Distribution of continuous variables was analyzed using semiquantitative parameters (kurtosis and asymmetry) and the Kolmogorov-Smirnov test. Normally distributed continuous variables are reported as mean ± standard deviations and were compared between groups with Student’s t-test; categorical variables are reported as counts (percentage) and were compared with Pearson’s χ2 or Fisher’s exact test for counts less than 5 in the contingency table.
Results
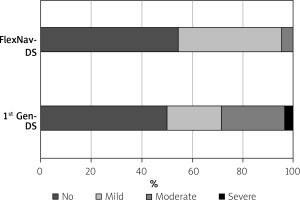
The study population consists of 50 patients, 28 in the 1st gen DS group and the remainder in the FlexNav DS group. Average age was 81.6 ±3.8 years and mean STS-PROM score was 5.2 ±2.8. The Heart Team recommended TAVR because of age, estimated risk for surgery, comorbidities, or previous heart surgery. The two groups were matched for age and, apart from female gender, did not differ in other clinical characteristics such as risk factors, body mass index, previous medical history, renal function and left ventricle ejection fraction (Table I). The indication for TAVR was severe aortic valve stenosis in all patients except one, in which a degenerated aortic bioprosthesis with severe regurgitation was treated. No patient had a bicuspid aortic valve. Procedural characteristics are reported in Table II. Most patients received balloon aortic valvuloplasty prior to valve implant; larger valves (n.27-29) were used in most patients and postdilatation was used in about one third of patients, with no significant differences between groups. The valve was successfully implanted in all patients except one of the 1st gen DS group in which a severe paravalvular leak was observed and treated by valve-in-valve implantation. Moderate-to-severe paravalvular leaks were more often observed in the 1st gen DS group as compared to the FlexNav DS group (28.6% vs. 4.5%; p = 0.03. Figure 1). In-hospital outcomes are reported in Table III. No patient died or needed bailout cardiac surgery. One patient in the 1st gen DS experienced a minor stroke. The rate of VARC-3 bleeding in the overall cohort was 16% although only 2 patients (one in each group) experienced type 2 bleeding whereas no patient experienced more severe bleeding. We observed 2 femoral pseudoaneurysms (1 in each group) which needed surgery and transfusion of 2 units of red blood cells and 2 large femoral hematomas needing transfusions in the 1st gen DS group; overall, VARC-3 adjudicated major vascular complications were more frequent, although not significantly, in the 1st gen DS group (10.7% vs. 4.5%; p = 0.64) as compared to the FlexNav DS group. The rate of permanent pacemaker implantation was higher in the FlexNav DS group (27.3% vs. 14.3%; p = 0.25) whereas the rate of new left bundle branch block was significantly lower as compared to 1st gen DS (9.1% vs. 35.7%; p = 0.03).
Table I
Clinical characteristics
Table II
Procedural characteristics
Table III
In-hospital outcomes
Discussion
In this single-center, retrospective analysis of prospectively collected data we compared in-hospital clinical outcomes after Portico TAVR with the FlexNav DS as compared to the 1st gen DS. The use of the new DS was associated with less frequent occurrence of moderate-to-severe paravalvular leak and of major vascular complications (the latter difference being not statistically significant) but with a higher, although not statistically significant, rate of permanent pacemaker implantation as compared to the previous delivery system. Despite the observational design, the 2 groups of patients were similar for most clinical characteristics except for a significantly higher prevalence of female gender in the FlexNav DS group. This difference might be relevant since, in most randomized trials and clinical registries, women undergoing TAVR are at higher risk of early vascular and bleeding complications as compared to men [7].
Technological improvement of transcatheter aortic valves is of paramount importance in order to provide new generation devices with the potential to increase procedural success, reduce complications and guarantee long-lasting results. These issues are critical since TAVR is moving from a prohibitive/high-risk population [8], mostly represented by elderly patients with limited life expectancy, to an intermediate-low risk (and younger) population [9, 10]. Vascular complications [11], paravalvular leaks [12] and conduction system disturbances [13] are still relatively frequent after TAVR and represent negative prognostic factors. In the case of the Portico heart valve system, the introduction of the FlexNav DS has represented a significant innovation with the potential to reduce vascular complications, mainly due to the lower profile, and to allow for a more precise, controlled delivery of the valve, possibly leading to higher implants and, in turn, to fewer paravalvular leaks and conduction system disturbances. Indeed, in a pooled analysis on 140 patients from the PORTICO IDE Trial and the FlexNav EU CE Mark study, the FlexNav DS was associated with a high device success rate, 5.0% rate of major vascular complications, 15.4% rate of permanent pacemaker implantation and 4.1% rate of moderate paravalvular leak [3]. An excellent safety and efficacy profile was also reported, albeit in a small Italian case series [4]. In our population, we observed a 4.5% rate for both major vascular complications (despite a higher prevalence of female patients) and moderate paravalvular leak with the FlexNav DS. Differently, in the 1st gen DS group the rate of more than mild paravalvular leak was disturbingly high (28.6%), although a severe paravalvular leak was only observed in 1 patient. Although a relatively high rate of more than mild paravalvular leak was reported with the Portico valve and the 1st gen DS as compared to the Sapien 3 Valve (8.2% vs. 4.5%, respectively) [14], possible further explanations for this finding might be represented by the higher, albeit non statistically significant, prevalence of moderate-to-severe pre-TAVR aortic regurgitation and of larger valve sizes in the 1st gen DS group as compared to the FlexNav DS group.
As far as conduction disturbances are concerned, we observed a higher rate of permanent pacemaker implantation but a lower, statistically significant rate of new left bundle branch block with the FlexNav DS. Unfortunately, the unavailability of data on valve implantation depth and on pre-existing conduction defects prevents us from making a reliable assessment of the impact of the FlexNav system on the development of conduction defects following TAVR, although the implementation of the stability layer could theoretically allow more precise control of valve release and implantation depth.
Our study has significant limitations, mainly represented by the single-center, observational design and the low number of patients enrolled. A possible confounder is the greater operator experience in the second period of the study, during which the patients in the FlexNav DS group were enrolled.
Conclusions
Our study, to the best of our knowledge, is the first comparison between the new FlexNav DS and the 1st Gen DS of the Portico heart valve system. Like previously published findings, our data show good performance of the FlexNav DS, particularly in terms of vascular complications and paravalvular leak.