Introduction
Shea trees (family Sapotaceae, Vitellaria paradoxa C.F. Gaertn) are economically important tree crops across several countries in sub-Saharan Africa spanning from Benin to Equatorial Guinea (Naughton et al., 2015). The genus Vitellaria consist of a single species, with two subspecies: subsp. nilotica is found in Sudan and Uganda, with small populations in Ethiopia and DR Congo, while Subsp. paradoxa occurs from Senegal to the Central African Republic (Byakagaba et al., 2011). In Ghana, shea trees grow wild in the Guinea and Sudan savannah agro-ecological zones (Western Dagomba, Southern Mamprusi, Western Gonja, Lawra, Tumu, Wa, and Nanumba), covering nearly all the areas of the northern part of the country, with sparse shea tree cover found in Brong, Ahafo, Ashanti, and Eastern and Volta regions in the south of the country (Jasaw et al., 2015). Thus, these trees cover over 77,670 square kilometers of land with about 94 million shea trees, which produce around 60 000 tons of shea nuts a year (FAO, 2018).
The shea crop is a local resource that provides continuous employment opportunities and generates income for women in the growing regions. Nutritionally, oil from the shea tree (shea butter) forms a chief constituent of fatty acids and glycerol in the diets of people of northern Ghana (Agyekwena, 2011). The ripe fleshy fruits are a good source of vitamins for indigenes and also serve as a food security crop as ripening coincides with the dry season when other food supplies have become scarce (Agyekwena, 2011; Aguzue et al., 2013). In the manufacturing, cosmetic, and rubber industries, shea butter is highly valued for the production of cakes, margarines, chocolates, cosmetics, soaps, candles, and rubber (Adazabra et al., 2013; Jasaw et al., 2015; Iddrisu et al., 2019). The tree also has therapeutic properties as it is used for the treatment of ailments, including dermatitis, rheumatism, ulcers, and body pain (Maanikuu and Peker, 2019). Ecologically, shea tree helps to improve soil fertility, sustains indigenous plant and animal biodiversity, and contains a considerable amount of carbon store that can be utilized via sequestration to mitigate climate change (Luedeling and Neufeldt, 2012).
While the economic, pharmaceutical, environmental, and other benefits of shea tree are incontrovertible, its propagation is plagued with a number of limitations. Traditionally, natural populations of shea tree are often left to stand when the land is cleared for the cultivation of staples such as legumes and cereals, with relatively little attention paid to its deliberate cultivation as a potential cash crop. Generally, new stands arise from nuts (seeds) that germinate on their own. The seeds have short viability, and germination is erratic (Lovett and Haq, 2013). This issue is exacerbated by the widespread collection of nuts for processing, thereby further limiting the regeneration of the crop through the use of seeds (Nyarko et al., 2012). Other constraints to successful shea tree cultivation are long juvenile phase, unavailability of improved genetic stocks, and lack of knowledge regarding efficient methods for the propagation of the species. Vegetative propagation is hindered by exudation of latex at the excised ends of twigs and branches, which interferes with rooting of cuttings. The use of grafting as a cultivation method is only recently yielding some results, though they are limited (Sanou and Lamien, 2011; Amissah et al., 2013). Pollination in shea is carried out by insects or wind, and thus produced plants are highly heterozygous (Nasare et al., 2019). Moreover, the long vegetative phase of the tree (between 10–15 years) in addition to the steady degradation to the ecological system has led to the destruction of wild, isolated shea trees or groves, hence posing a threat to commercialscale propagation and domestication of shea tree (Honfo et al., 2014; Bello-Bravo et al., 2015). To address these constraints, there is a need to find an alternative approach to improve propagation and enhance large-scale cultivation of shea tree. Explant culture methods have shown success in the propagation of recalcitrant tree crops and woody plants (Singh et al., 2014). Nanti et al. (2020) and Dickson et al. (2011) reported the regeneration of cashew and rubber by using shoot tip and embryo explants, respectively. Although micro-propagation protocols have been documented in some species of the Sapotaceae family (Bhore and Preveena, 2011; Silveira et al., 2016; Kunwar and Thakur, 2017; Amghar et al., 2021), limited attempts have been made for shea, except for a few reports (Fotso et al., 2008; Adu-Gyamfi et al., 2012; Issali et al., 2013; Lovett and Haq, 2013), all of which reported very low rate of regeneration. The present study investigated the effect of different concentrations of BAP, KIN, and NAA on shoot and root development in vitro through shoot-tip induction to provide good quality shea cuttings.
Materials and methods
Preparation of explant and surface sterilization
Six-week-old shoots were sourced using sharp knives from a previously decapitated shea tree at the Biotechnology Centre of the Biotechnology and Nuclear Agriculture Research Institute (BNARI), Accra, Ghana. Excised shoots were either pink or green in color. They were trimmed of older leaves, leaving 1–2 leaf primordia per explant. Explants were then gently washed using liquid soap and left under the running tap water for 1 h. The explants were then transferred into oven-dried jars for aseptic sterilization. Two steps of the sterilization protocol were carried out under laminar air flowhood conditions; explants were initially sterilized with 0.2% HgCl2 for 5 min, followed by 0.1% HgCl2 for 3 min, respectively. After each stage of surface sterilization, explants were thoroughly washed three times with sterile distilled water and trimmed to the required size for culture.
Culture medium
Surface-sterilized shoot explants were inoculated onto 40 ml of Murashige and Skoog, 1962 (MS) medium supplemented with 3% sucrose, cytokinins (BAP or KIN alone; Sigma Aldrich, Germany) at the concentrations of 0 (control) to 2 mg ∙ dm−3 or a combination of cytokinins (BAP and KIN at the concentrations of 0.25 to 0.5 mg ∙ dm−3) and auxin (NAA) at a constant concentration of 0.5 mg ∙ dm−3 in 250 ml jars. Prior to the addition of phytagel (3.5 mg ∙ dm−3; Sigma Aldrich, Germany), the pH of the medium was adjusted to 5.8. The medium was then autoclaved at 121°C for 15 min and pressure of 15 psi. The cultures were kept in the growth room at 25°C and 16 h light/8 h darkness using white florescent tubes at 50 μmolm-2 ∙ s-1 (CRORCH, China) and relative humidity of 90%.
Collection of experimental data and statistical analysis
The cultures were observed every day for signs of growth. Data were recorded regarding days to shoot and root induction, percentage survival, number of leaves, and shoot height at 2, 4, and 6 weeks after culture. The experimental design was Completely Randomized Design (CRD) with three replicates per treatment. Each replicate consisted of 10 explants. Data collected were analyzed using the GenStat statistical software program (11th edition). ANOVA was used to determine the differences between treatment means. Mean values were compared and analyzed by Tukey’s pairwise comparison.
Results
Effect of BAP or KIN on shoot regeneration
Shoot tip explants of shea incubated on MS medium supplemented with varying concentrations of BAP or KIN (0, 0.5, 1.0, 1.5, and 2.0 mg · dm−3) sprouted within 8 to 29 days after culture (Table 1A and Table 1B; Fig. 1B). The maximum number of explants initiated when MS medium was supplemented with 1.5 mg · dm−3 KIN was 30 (100%), and the minimum was 3 in MS without growth regulators (control treatment) (Table 1A and Table 1B). The highest number of days (29 days) required for shoot formation was observed in the control plant. However, the period was significantly (P < 0.05) reduced to 8 days when the culture medium was supplemented with 2 mg· dm−3 KIN (Table 1A and Table 1B). Furthermore, the number of days to shoot emergence depended on which growth regulator was used. The minimum number of days for shoot induction in BAPmodified medium was recorded at 0.5 mg· dm−3, while in the case of KIN, a concentration of 2 mg · dm−3 recorded the least number of days to shoot induction. In general, shoot tip explants cultured in vitro were able to regenerate shoots of shea in all the concentrations of BAP or KIN tested. The results of ANOVA on shoot regeneration showed that the percentage explants producing shoots were significantly influenced by the inclusion of BAP or KIN in the culture medium. Cultures on growth regulator-free medium showed the lowest percentage for shoot induction (10%). Optimum concentrations for BAP and KIN were 0.5 mg · dm−3 BAP and 1.5 mg · dm−3 KIN, as these induced 80 and 100% shoot emergence of cultured explants after 4 weeks of culture, respectively (Table 1A and Table 1B). However, of the two cytokinins, BAP showed better results in terms of shoot vigor than KIN (Fig. 1C and Fig. 1D). The average length of the longest shoot was recorded as 2.76 cm on the medium supplemented with 1.5 mg · dm−3 BAP, whereas the shortest (1.40 cm) was observed at 2 mg · dm−3 BAP. By increasing or decreasing the concentration of BAP or KIN, percent shoot regeneration and shoot length decreased below the optimum value (Table 1A and Table 1B).
Fig. 1
In vitro regeneration of shea. A) inoculated shoot tip; B) shoot emergence after 8 days of culture; C) shoot developing on MS + 1.5 mg · dm−3 KIN; D) shoot developing on MS + 0.5 mg · dm−3 KIN; E) root induction at the base of plant; F) root induction around the stem of explant

Table 1A
The effect of BAP [mg · dm−3] on micropropagation of shea
Table 1B
The effect of KIN [mg · dm−3] on micropropagation of shea
The combination of BAP/NAA, KIN/NAA, and KIN/BAP also showed good results for shoot formation (Table 2A and Table 2B). Among these combinations, 2 mg · dm−3 KIN + 0.5 mg · dm−3 NAA showed significantly higher (P < 0.05) shoot regeneration (100%) and longer shoot height (3.24 cm), although time taken for shoot induction was longer (7 days) than that for the medium supplemented with 1.5 mg · dm−3 BAP + + 0.25 mg · dm−3 KIN that recorded the lowest number of days (6) for shoot induction (Table 2A and Table 2B). The combinations 1.5 mg · dm−3 BAP + 0.25 mg · dm−3 KIN, 1 mg · dm−3 KIN + 0.25 mg · dm−3 BAP, and 1.5 mg · dm−3 KIN + 0.25 mg · dm−3 BAP also showed high shoot regeneration (80%). For root formation, none of the combinations tested could induce root except the medium supplemented with 1 mg · dm−3 BAP + 0.5 mg · dm−3 NAA, which induced 3.40 mean roots (53.33%) after 46 days of culture (Table 2b). Two types of adventitious roots were observed: 1) thick creamy roots formed directly at the cut surface of explant and 2) indirect roots through brownish callus formed around the lower stem. The roots were brownish and thread-like (Fig. 1E and Fig. 1F).
Table 2A
The effect of KIN, NAA and BAP [mg · dm−3] on propagation of shoots and roots of shea in vitro
Table 2B
The effect of BAP, NAA and KIN [mg · dm−3] on propagation of shoots and roots of shea in vitro
Effect of plant growth regulators and age of culture on shoot regeneration
The response of shoot tip explants to varying concentrations of BAP or KIN alone or in combination with NAA or KIN/BAP showed varied results in relation to shoot and leaf development at 2, 4, 6, and 8 weeks of culture (Fig. 2). Leaf formation was observed on MS medium supplemented with all tested plant growth regulators irrespective of the age of culture, except at 1 mg · dm−3 BAP for week two where no leaf was formed. However, cultures of explants on MS medium devoid of plant growth regulators (control) developed leaves only after 8 weeks (Fig. 2). The type and concentration of cytokinins and/or auxin influenced the average number of leaves produced per explant and the mean shoot height. For medium containing BAP alone, the maximum average number of leaves (2.9) and shoot height (2.96 cm) was observed at 1 mg · dm−3 concentration, while 1.5 mg · dm−3 KIN recorded the highest values for the number of leaves (1.7) and shoot height (2.57 cm) (Fig. 2).
Fig. 2
Effect of different concentrations of plant ygrowth regulators on shoot and leaf formation of shea tree in vitro. A) mean number of leaves after 2, 4, 6, and 8 weeks of culture; B) plant height after 2, 4, 6, and 8 weeks of culture; vertical bars at the top represent standard errors
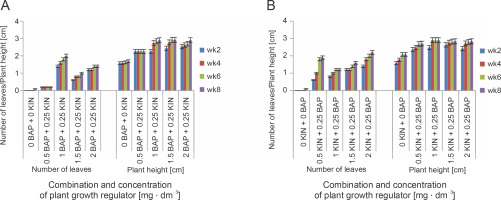
Explants cultured on different concentrations and in combination with BAP, KIN, or NAA showed the highest number of leaves and shoot height at 8 weeks after culture, although the media containing 1.5 mg· dm−3 BAP + 0.5 mg · dm−3 NAA recorded the lowest number of leaves (0.4) and shoot height (2 cm) at week 8 (Fig. 2). Moreover, although all the various growth regulators/combinations could regenerate shoots from shoot tip explants, 2 mg · dm−3 KIN combined with 0.5 mg · dm−3 NAA was the most effective in producing the highest mean number of leaves (4.2) and shoot height (4 cm) (Fig. 2). Generally, the average number of leaves and shoot height increased with the age of culture, except when explants were cultured on the medium supplemented with 0.5 mg · dm−3 BAP + 0.25 mg · dm−3 KIN, which showed no growth irrespective of the age of culture (Fig. 2). Besides, the mean values observed for leaf number and shoot height at 6 weeks after culture did not differ substantially from those of week eight. After 8 weeks of culture, most plants lost vigor in growth, which was characterized by leaf defoliation, and in some cases, the death of cultures (Fig. 3).
Discussion
This experiment was an attempt to achieve efficient regeneration of shea tree plantlets by using shoot tip explants. Explants inoculated on MS medium supplemented with different types and varying concentrations of cytokinins significantly improved shoot induction over the controls. Optimal shoot morphogenesis was obtained on 1.5 mg · dm−3 KIN-supplemented medium; at this concentration, 100% shoot regeneration was observed. However, days to shoot induction and shoot length were best on medium supplemented with 2 mg · dm−3 KIN and 1.5 mg · dm−3 BAP, respectively.
Plant growth regulators play a vital role in the growth and development of plants; cytokinins including BAP and KIN are known to enhance cell division and the development of both adventitious and axillary shoots in plants (Jana et al., 2013; Kumlay and Ercisli, 2015; Kodad et al., 2021). This may explain why the control (which contained no growth regulators) took a comparatively longer time for shoot initiation, and the lowest mean values for shoot induction and shoot height were recorded for this plant. Thomson and Deering (2011) and Pandey and Tamta (2015) reported the best regeneration response on MS supplemented with KIN in Corylus avellana and Quercus leucotrichophora L., respectively. Darwesh et al. (2017) and Lamaou et al. (2018) showed best shoot induction response on MS medium containing BAP in Argania spinosa L. and Kaya senegalenses, respectively. The different results obtained by these authors may be ascribed to genotypic differences among the different plants. Moreover, the decreasing mean values obtained for percentage shoot regeneration and shoot length beyond the optimum concentration may be a result of the inhibitory effect of the plant growth regulators at higher concentrations (Demeke et al., 2014). Generally, increasing concentrations of plant growth regulators correlate with an increasing physiological response of cultures until the plant reaches a “saturation point,” beyond which the application of higher concentrations of plant growth regulators results in a negative physiological response (Hussien et al., 2011). Similar findings were reported by Abu-Romman et al. (2015), who suggested that applying higher concentrations of plant growth regulators beyond an optimum level decreases the physiological response of in vitro plants.
The combinations of cytokinin(s) and/or auxin also improved shoot and root induction. Evaluations indicated that the combination of 2 mg · dm−3 KIN + + 0.5 mg · dm−3 NAA was significantly the most efficient at shoot regeneration and shoot elongation compared to the remaining tested conditions. Consequently, this was noted as the best treatment for highest shoot induction among all the other treatments.
The auxin/cytokinin combination has been known to signal molecules that control growth and development. According to Fatima et al. (2011), high concentrations of cytokinins along with low concentrations of auxins synergistically affect in vitro plant regeneration and cell division. In parallel to our findings, some earlier researchers have also reported on morphogenic responses among the members of the Sapotaceae family by using various explants and different plant growth regulators. Salud et al. (2017) and Sanonne et al. (2013) reported on the successful regeneration of Argania spinosa and Baillonella toxisperma through seed and shoot tip explants, respectively. For shea tree, Adu-Gyamfi et al. (2012) sprouted somatic embryos using immature cotyledon explants on MS medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), while Lovett et al. (2013) reported shoot regeneration on MS medium supplemented with BA and NAA through shoot tip explants.
Although the rooting of micro shoots in woody trees is considered problematic because of poor development and low rate of rooting (Sharma et al., 2017), the use of auxins (such as NAA, IBA, and IAA) singly or in combination with cytokinins (such as BAP and KIN) for root regeneration has been reported by other workers (Anis et al, 2010; Venkatachalam et al., 2015; Alelegne et al., 2020). For Sapotaceae species, different types and concentrations of auxin/cytokinin ratios have been reported. For instance, 2 mg · dm−3 IBA + 0.5 mg · dm−3 BAP showed best root response in miracle berry (Synsepalum dulcificum) (Ogunsola and Ilori, 2008), while a range of 4.9 to 14.8 mg · dm−3 IBA showed the best response in shea (Lovett et al., 2013). However, in our study, the combination of 1 mg· dm−3 BAP + 0.5 mg · dm−3 NAA induced the best root formation. The variation in results obtained by different authors could be attributed to different cytokinin/auxin ratios used and genotypic differences among shea explants used for the various studies.
Conclusion
The study reveals variations in behavior of tissue-cultured explants of shea (V. paradoxa ) to varied concentrations and different types (either singly or in combination) of plant growth regulators. Of 24 growth regulator treatments tested, the best shoot regeneration response was observed on MS medium supplemented with 2 mg · dm−3 KIN + 0.5 mg · dm−3 NAA or 1.5 mg · dm−3 KIN. However, the cytokinin BAP showed vigorous shoot growth than KIN. Rooting was induced only on MS medium modified with 1 mg · dm−3 BAP + + 0.5 mg · dm−3 NAA. Despite these findings, the process of root and shoot regeneration needs to be optimized for routine regeneration of the crop.











