Trial registration: clinicaltrials.gov (NCT03976726).
The i-gel mask (Intersurgical, Wokingham, UK), is a second-generation, single-use supraglottic airway device. In contrast to other devices, its cuff consists of a thermoplastic elastomer (styrene-ethylene-butadiene-styrene) instead of an inflatable cuff.
Previous studies have shown that over time the sealing improved over a period of 10 minutes compared to the moment of insertion [1, 2]. The sealing process may be based on various mechanisms. It is assumed that the warming from room to body temperature leads to improved adaptation to the patient-specific anatomy due to softening of the elastomer [1, 3, 4]. By definition, an elastomer is softened by the effect of temperature and becomes harder when reaching the glass transition temperature.
In this context a change in the degree of hardness as a function of temperature had been described before [5]. Prewarmed i-gel masks (42°C, 30 min) seem to have smaller leak volumes compared to masks stored at room temperature postinsertion in paralyzed patients [6], but this was not reproducible in non-paralyzed patients [7–9]. Looking at the sealing pressure it was reported that i-gel masks required a lower pressure compared to masks with an inflatable cuff [3].
At this point it is not clear whether the thermoplastic cuff material only expands due to an increase in temperature. Liquid absorption and a consecutive expansion are also theoretically conceivable.
Therefore, the aim of the present study is to examine the i-gel mask within the scope of a benchtop study for temperature-dependent volume expansion and liquid absorption.
METHODS
After consultation and approval by the local Ethics Committee University Hospital Frankfurt, Frankfurt, Germany (Chairperson Prof. Dr. Harder) on 16th January 2019 there was no need to obtain written informed consent from the patients. All masks were examined after use. No patient was influenced by this investigation. All investigations were carried out in accordance with the Declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT03976726).
At first, masks were examined in a benchtop study. Data thus obtained were then compared with masks used in patients with regard to comparability and reproducibility of volume and weight increase as well as changes in density.
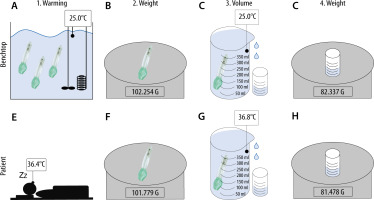
Figure 1 represents the study setup. Figure 1A–D shows the sequence of the benchtop study, while Figure 1E–H illustrates the measurement process of the masks used in patients.
FIGURE 1
Setup/Flowchart. Benchtop: A) Warming i-gel in a water bath. B) Determine weight of dry masks. C) Collecting the overflow water. D) Determining the weight of the overflow water. Patient: E) Using i-gel in a patient. Determining weight of dry masks. F) Collecting the over flow water. G) Collecting the overflow water. H) Determining the weight of the overflow water
Benchtop measurement
Three masks of sizes 3, 4 and 5 were evaluated. All masks were submerged in a water bath (WNB 10, memmert GmbH&Co.KG, Schwabach, Germany) and remained for at least 10 minutes per temperature step to equilibrate (Figure 1A). This period was chosen according to Lee et al. [2]. The water bath contained a thermostatically controlled heating element and the water was stirred continuously. For measurements in the lower temperature range, ice was added to the water bath to achieve the desired target temperature. All measured values for a temperature range from 10°C to 50°C were determined in steps of 5°C each. As shown in Figure 1A–D, the sequence of a measurement process was structured as follows:
One mask was taken out and immediately dried by two blasts of compressed air to prevent the masks from cooling down. Directly after drying the weight was determined (Figure 1B). The mask was then submerged in a beaker filled with water at the same temperature as the water bath. The overflowing water was collected, and its weight determined by a digital laboratory balance (ABJ 220-4M, Kern & Sohn GmbH, Balingen, Germany) to three decimal places (Figure 1C). Since the density (ρ) of water at certain temperatures is known [10], the volume (V) of the displaced water could be calculated from the weight (m) by the following equation: V = m × ρ-1 (Figure 1D). This volume is identical to the mask investigated. This measurement was repeated three times per temperature step and mask. Therefore, the mean value was calculated. In total, this measurement process was carried out for all nine masks and the results of each of the three masks of the same size were also averaged (Table 3).
TABLE 1
Patient characteristics and use of i-gel
TABLE 2
Equations of regression lines for volume and density
TABLE 3
Differences of i-gel between room temperature and body temperature
Clinical investigation
Masks used in patients (n = 5 per size) requiring general anaesthesia for elective surgery (arthroscopy (hand or knee), metal removal, osteosynthesis of distal bones (e.g. distal radius, ankle joint)) without contraindications for i-gel were collected postoperatively. Demographic data and duration of i-gel use are depicted in Table 1.
An appropriately sized i-gel was chosen based on the patient’s total body mass as per the manufacturer’s instructions (Figure 1E) [11]. Continuous esophageal measurement of body temperature was carried out using a temperature probe (Rüsch Rectal/Pharyngeal Temperature Sensor REF 1016, Teleflex Medical, Athlone, Ireland) via the suction duct. After extubation, the mask was dried immediately by two blasts of compressed air, and its weight determined (Figure 1F). The volume was measured according to the above-mentioned procedure (Figure 1G–H). After the used mask cooled down to room temperature, weight, volume and density were obtained again (Figure 1F–H).
Comparison between benchtop and patient measurement
In order to compare the results of the measurements of masks used in patients with those of the benchtop examination, volume and density were examined for correlation. For this purpose, these parameters were calculated for the respective measured room and body temperature using the equation of the regression lines. The results were then compared using Pearson correlation.
Statistical analysis
Results were collected and stored in a Microsoft Excel (Microsoft Office 365, 2016) database. The sta-tistical analyses were performed using SigmaPlot 12 (Systat Software GmbH, Erkrath, Germany). For descriptive analysis mean values were calculated. Values were expressed as number (count) and percentage, as appropriate. The Shapiro-Wilk test was used to test for normal distribution of the data. To detect differences Students’ t-test or the Mann-Whitney U test was used. Linear correlation analysis was performed to determine the relationship between the volume and density of masks used in patients and the benchtop study. Pearson’s correlation coefficient R and determination coefficient R2 were computed as the ratio of covariance between the variables to the product of their standard variation. A post-hoc power analysis was performed, revealing a power of 0.999 for n = 5 masks used in patients. The level of significance was set to P < 0.05.
RESULTS
The volume and weight of all masks were measured, and the density was calculated.
Results of the benchtop measurement are shown in Figure 2 as line-connected dots. Results of in patient used masks are shown as single results in Figure 2A–C. Red circles represent values obtained at body temperature, red squares results obtained at room temperature. Thee equations of the regression lines, if applicable, are depicted in Table 2. The results are split according to mask sizes (size 3 = Figure 2A, D, G; size 4 = Figure 2B, E, H; size 5 = Figure 2C, F, I).
FIGURE 2
Columns represent results of i-gel masks with identical size (size 3 = A, D and G; 4 = B, E and H; 5 = C, F and I). Rows represent volume (= A, B and C), weight (= D, E and F) and density (= G, H and I) from top to bottom. The X-axes are indicated as temperature in degrees Celsius. The Y-axes are specified differently depending on the variables displayed. Squares represent the measurement results at room temperature, while circles represent the results at body temperature (n = 5 per size). Black lines (if depicted) represent the regression lines, while colored lines connect the individual measurement results. Dot connected lines represent the results from the benchtop study (n = 3 per size)

Volume
The volume increased linearly with higher temperature. Patient-used i-gel masks of size 3 increased by 0.10 mL per degree Celsius, size 4 masks by 0.13 mL and size 5 masks by 0.07 mL.
Volume increase in all masks reached the level of significance (Table 3). The mean volume expansion of in patient-used masks of size 3 masks was 1.42 mL (= 1.97%; SD = 0.16; DTemp. = 14.8°C; P < 0.001), size 4 masks 1.78 mL (= 2.01%; SD = 0.16; DTemp. = 14.2°C; P < 0.001) and size 5 masks 0.95 mL (= 0.86%; SD = 0.13; DTemp. = 14.4°C; P < 0.001). The results showed a very strong correlation with the regression line (size 3: R2 = 0.980; size 4: R2 = 0.984; size 5: R2 = 0.993).
Weight
The weight increased non-linearly with higher temperature. All masks, regardless of size, showed a weight gain up to a temperature of 36°C in the benchtop measurements. This was followed by a short decrease with a low point at 40°C, followed by a stronger temperature-dependent weight increase as shown in Figure 2D–F. This results in a significant increase in weight in the patient measurements at a temperature range of rounded 22°C to 36.5°C. Size 3 masks increased their weight by 21.0 mg (= 0.03%; SD = 0.008; P = 0.018), size 4 masks by 31.0 mg (= 0.04%; SD = 0.024; P = 0.027) and size 5 masks by 53.6 mg (= 0.05%; SD = 0.042; P = 0.006).
Density
The density decreased linearly with higher temperature. In patient-used i-gel size 3 masks density decreased by 0.13% per degree Celsius, size 4 masks by 0.14% and size 5 masks by 0.06%. In the investigated temperature range within the patient measurements the density of size 3 masks decreased significantly by 0.02 γ cm-3 (–1.90%; SD = 0.002; P < 0.001), in size 4 by 0.02 γ cm-3 (–1.93%; SD = 0.002; P < 0.001) and in size 5 by 0.01 γ cm-3 (–0.80%; SD = 0.001; P < 0.001). The results showed a very strong correlation with the regression line (size 3: R2 = 0.970; size 4: R2 = 0.965; size 5: R2 = 0.987).
Comparison between benchtop and patient measurement
There was a strong correlation between the results of the benchtop examination and the values obtained from the masks used in patients.
The results between benchtop and patient measurement showed a strong correlation for volume calculation at both room (R = 0.999; R2 = 0.999; P < 0.001) and body temperature (R = 0.999; R2 = 0.999; P < 0.001). Also strong correlations were found with regard to the determination of density (R = 0.996; R2 = 0.992; P < 0.001) and body temperature (R = 0.914; R2 = 0.835; P < 0.001).
DISCUSSION
The present study revealed in all i-gel masks (sizes 3, 4 and 5) a significant temperature-dependent increase in volume and weight as well as a significant decrease in density. All results showed a strong correlation between benchtop and patient measurements. These results represent a new approach to explain how the i-gel improves its sealing over time.
The sealing process of the i-gel mask, with its cuff consisting of a thermoplastic elastomer, is based on a mechanism that has not been conclusively clarified. Several theories exist that could explain this process. A widespread idea is that the increase in temperature caused by heating from room to body temperature leads to increasing moldability. This could lead to better adaptation of the mask to anatomical structures, and it was shown that there is a potential deformation due to softening of the elastomer. It has been shown that there is a temperature-dependent change in hardness and resistance. Higher temperatures were associated with increased deformability [5].
In addition to changes in physical properties, a lower sealing pressure was observed when i-gel masks were used compared to LMA supreme [12]. In contrast, another group did not find any significant difference in sealing pressure when comparing i-gel with LMA supreme, although significantly greater leakage was observed in patients with i-gel [13]. This leakage was explained by potentially poorer (initial) sealing due to inadequate adaptation to anatomical structures. However, it seems very likely that the i-gel changes its properties due to the influence of temperature. Thus, the leakage is reduced within the first 10 minutes, which corresponds to a temperature adjustment from room temperature to body temperature, and has already been reported by several authors [1, 3, 4, 12].
The properties investigated in this study were the volume, weight and density change of the masks in a benchtop examination and subsequently in the patient.
The coefficient of expansion of styrene-ethylene-butadiene-styrene [14] is 16 x 10-5 x K-1. Therefore, a significant expansion of the cuff should theoretically not occur when the temperature increases from room to body temperature. However, our results show otherwise. A significant increase in volume (P < 0.001) and weight (3: P = 0.018; 4: P = 0.027; 5: P = 0.006) and a decrease in density (P < 0.001) were observed. A possible explanation for this, besides expansion, is the absorption of fluids, which was also discussed before [5]. The wet milieu, caused by the saliva, could be responsible for this. Looking at the weight changes of the masks, it was found that the initial weight remained almost constant. Only at temperatures of 40°C and above did the weight increase. This suggests that liquid absorption only occurred at higher temperatures. Here temperature-dependent pore opening or increasing porosity would be a possible explanation. Since this circumstance only occurred from temperatures of 40°C, it does not seem to be relevant for the daily routine at normal body temperatures. We investigated a temperature range from 10°C to 50°C. We chose this temperature range due to multiple reasons. Often there are relevant changes under extreme conditions. Furthermore, it was important to us that our results were comparable with already published data. A previous study investigated the physical properties of i-gel masks in a temperature range from 10°C to 60°C [5]. However, since these are not only in vivo but also in vitro examinations, we chose temperatures over body temperature. In case i-gel masks are used in emergency medicine the masks may be stored in ambulances during summer/winter. In ambulances, high temperature fluctuations can occur due to the weather, which can lead to the use of i-gel masks in extreme temperature ranges. However, since the masks are stored in their original packaging and not in a liquid, it is questionable whether there would be changes in the variables investigated.
The volume expansion we observed was unimpeded in the benchtop setting. The placement of the mask in the hypopharynx makes expansion through the anatomically restricted space more difficult. In order to investigate this circumstance more closely, we compared the results of our benchtop study to the results of the patient measurements. There were strong correlations in all parameters obtained at room temperature as well as at body temperature. However, this setting does not take into account whether the change in volume and degree of hardness in the hypopharynx improve the sealing and whether the mask really adapts better to anatomical structures. Further investigations are necessary to evaluate the increase in volume in a restricted setting.
CONCLUSIONS
To the best of our knowledge, the present study is the first to investigate the volume expansion, weight and density change of the i-gel mask. We were able to identify significant changes in all three properties investigated, which represent a further component in explaining the mechanism how the i-gel P functions. It can therefore be assumed that it is not a single effect, but rather the sum of many different effects that explain the functioning of the i-gel mask.




