Introduction
Asthma is a chronic inflammatory disorder of the airways that may affect many million people globally and remains a health problem with significant morbidity and mortality. Hallmarks of asthma are inflammation of the airways leading to bronchial hyperresponsiveness and a variable degree in airflow obstruction. Structural changes of the bronchial wall which occur in asthma, mainly angiogenesis, vascular leak, smooth muscle hypertrophy and subepithelial fibrosis, contribute to airway remodelling. Moreover, in this process various important agents, like growth factors (e.g. vascular endothelial growth factor) and active cells (e.g. neutrophils) might be involved [1, 2].
Vascular endothelial growth factor (VEGF) is a key regulator of vascular development and vessel function not only in asthma, but also in carcinogenesis, chronic obstructive lung disease, age-related macular degeneration, chronic heart disease and rheumatoid diseases [3, 4]. VEGF is described as a homodimeric, heparin-binding glycoprotein with a molecular weight of 46 kDa and gene located on 6p21.3. Various reports suggest that platelets, epithelial cells, neutrophils and macrophages have the ability to produce VEGF which acts biologically by tyrosine-kinases receptors [5]. In asthmatics, VEGF plays an important role in Th2-type inflammatory responses, enhances allergic sensitization, promotes proliferation and differentiation of endothelial cells and intensifies chemotaxis of monocytes and eosinophils. VEGF-inhibitors and VEGF-receptors’ blockers might be a new therapeutic approach to control chronic airway inflammation and vascular remodelling [6, 7].
Neutrophil granulocytes are responsible for immune reactions during host-pathogen interaction and can react with various defence strategies like apoptosis, degranulation, phagocytosis and form neutrophil extracellular traps (NETs). Neutrophils mature in the bone marrow, next are released into the blood stream and may be stored in a marginal pool on blood vessel walls with a short lifespan with circulating half-lives of approximately 4–19 h [8]. Cells transmigration through the endothelium and basal membrane of blood vessels is guided by chemotactic agents such as bacterial components (N-formyl-methionine-leucyl-phenylalanine; fMLP), complement factors or chemokines [9]. Moreover, neutrophil-derived cytokines can be involved in the angiogenesis process – a potential role of VEGF, fibroblast growth factor 2 (FGF2), angiopoietin 1 (Ang1) and interleukin 17 (IL-17) remains to be elucidated [10]. Nevertheless, these mechanisms (transmigration and cytokine release) also can lead to destruction of healthy surrounding tissue what can explain the two-sided role of neutrophils in diseases, among others in asthma. Neutrophilic component is closely implicated in the underlying pathophysiology of severe, corticosteroid and refractory asthma and airway neutrophils might be characteristically elevated in asthma exacerbations. It is considered that neutrophil protease-mediated activation of airway epithelial cells and goblet cell degranulation in combination with neutrophil-mediated oxidative stress are possible mechanisms by which these cells decrease lung function in asthmatics [11]. Undoubtedly neutrophils are a heterogeneous cell population and a more precise phenotyping would help delineate different subtypes of asthma [12]. Detection of activated neutrophils by indication of different protein markers on the membrane using the flow cytometry might be a useful diagnostic tool.
Aim
In the present study, we investigated the expression of CD69 and CD11b markers on peripheral neutrophils unstimulated, after fMLP stimulation and after VEGF in vitro stimulation in patients with asthma. Furthermore, the possible influence of a genetic factor (del/ins genotype at -2549 -2567 position in the promoter of the VEGF gene) was taken into account. In the current literature, there are no data about this phenomenon.
Material and methods
Examined groups
The study population included a total number of 122 participants (aged from 20 to 70 years (mean ± SD: 50.56 ±12.57); 42 (34.43%) males) who gave written and informed consent for participation. In this group, 82 patients (aged from 23 to 69 years (mean ± SD: 51.92 ±12.17); 28 (34.15%) males) had a diagnosis of asthma according to criteria defined in The Global Initiative for Asthma (GINA) report [13]. Among asthmatics, 64 patients (78.05%) were treated with inhaled corticosteroids, 66 (80.49%) patients used long-acting β-mimetics and everyone (100%) declared to use short-acting β-mimetics. None of asthmatics used systemic corticosteroids during the study. The control group consisted of 40 subjects (aged from 20 to 70 years (mean ± SD: 47.95 ±13.66); 14 (35%) males) who were healthy, without positive medical history toward allergy or chronic pulmonary disease. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee at the Wroclaw Medical University, Poland.
Flow cytometric analysis
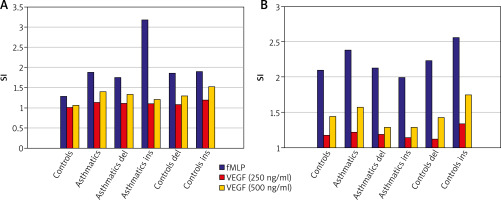
Peripheral blood neutrophils were suspended in medium (non-stimulated negative control – patient background: Pb), N-Formylmethionyl-leucyl-phenylalanine (fMLP in concentration of 10–6 M – positive control: Pc) or VEGF (two samples in concentration: 250 ng/ml and 500 ng/ml – selected experimentally). All samples contained 100 µl blood taken to the 4.5 ml tubes with lithium heparin (Sarstedt AG & Co., Nümbrecht, Germany) and 100 µl RPMI-1640 Medium (Institute of Immunology and Experimental Therapy, Wroclaw, Poland), fMLP (Institute of Immunology and Experimental Therapy, Wroclaw, Poland) or VEGF (BD Biosciences Pharmingen, San Diego, USA). Samples were incubated in the atmosphere supplemented with 5% CO2 at 37°C for 60 min (Incubator ASSAB, Stockholm, Sweden). Then, 20 µl of edentate disodium EDTA (BD Biosciences Pharmingen, San Diego, USA) was added and samples were centrifuged at 1600 rev/min for 10 min. In the next step, the supernatant was removed, 100 µl of PBS (Institute of Immunology and Experimental Therapy, Wroclaw, Poland) with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, USA) and 10 µl of anti-CD69 and anti-CD11b (Immunotech S.A.S., Marseille, France) were added and incubated in the darkness in 25°C for 30 min. After incubation, 2 µl fluid for the cell’s lysis (BD Biosciences Pharmingen, San Diego, USA) was added and after 10 min. at room temperature the samples were centrifuged at 1600 rev/min for 5 min. Afterwards, the supernatant was removed and to each sample 3 ml of PBS was added and centrifuged 5 min at room temperature at 1600 rev/min. To preserve the cells, 200 µl of PBS with 1.5% paraformaldehyde (Sigma-Aldrich, St. Louis, USA) was added. From each examined sample, cells were collected using a FACScan flow cytometer (Becton Dickinson, San Diego, USA). Briefly, to detect whether the cell antigen CD69 and CD11b expression on neutrophils surface was induced, specific fluorescence of the active neutrophils population was identified. The obtained results were further analysed as median with minimum and maximum values (Me (min.; max.)) of samples intensity (Tables 1). Findings were presented (Figures 1 A, B) as Stimulation index (SI), which is the quotient of fluorescence intensity after stimulation to fluorescence intensity of the primary sample without exogenous stimulation (negative control). Procedures of neutrophils identification described above were coincident with a widely accepted method [14].
Table 1
CD69 and CD11b expression on neutrophils in examined groups – values shown as a specific fluorescence (Me (min.; max.))
| Base | Asthmatics | Controls | Asthmatics del phenotype | Asthmatics ins phenotype | Controls del phenotype | Controls ins phenotype |
|---|---|---|---|---|---|---|
| CD69 expression: | ||||||
| Patient background (Pb – medium) | 257.62 (15.34; 516.12) | 225.22 (92.00; 514.46) | 233.32 (66.35; 516.12) | 295.59 (15.34; 439.21) | 221.99 (94.03; 514.46) | 233.51 (92.00; 401.92) |
| Positive control (Pc – fMLP) | 389.52 (54.98; 552.03) | 364.99 (160.92; 516.83) | 379.47 (168.87; 552.03) | 410.50 (54.98; 534.75)* | 380.22 (261.94; 514.85) | 345.55 (160.92; 516.83)* |
| VEGF (250 ng/ml) | 287.15 (15.41; 543.36) | 252.24 (112.72; 540.73) | 284.870 (80.76; 543.36) | 304.52 (15.41; 408.47) | 243.46 (115.31; 456.35) | 263.45 (112.72; 540.73) |
| VEGF (500 ng/ml) | 315.36 (15.06; 514.96) | 301.32 (105.35; 675.53) | 321.73 (99.28; 514.96) | 295.70 (15.06; 430.47) | 299.51 (105.35; 483.37) | 303.19 (133.51; 675.53) |
| CD11b expression: | ||||||
| Patient background (Pb – medium) | 663.93 (24.81; 1400.52) | 567.510 (172.75; 1433.9) | 233.32 (66.35; 516.12) | 785.41 (24.81; 1200.06) | 567.51 (196.23; 1433.96) | 603.54 (172.75; 1245.45) |
| Positive control (Pc – fMLP) | 1158.05 (150.44; 1687.93) | 1075.53 (458.27; 1562.52) | 379.47 (168.87; 552.03) | 1214.66 (150.44; 1588.16)† | 1095.08 (722.37; 1492.72) | 1063.79 (458.27; 1562.52)† |
| VEGF (250 ng/ml) | 787.38 (10.17; 1571.58) | 722.23 (229.79; 1505.81) | 284.87 (80.76; 543.36) | 827.59 (21.72; 1190.77) | 722.23 (229.95; 1268.37) | 725.01 (229.79; 1505.81) |
| VEGF (500 ng/ml) | 865.06 (21.72; 1433.59) | 818.21 (306.25; 1895.62) | 321.73 (99.28; 514.96) | 840.59 (21.72; 1190.77) | 798.88 (315.48; 1408.68) | 818.21 (306.25; 1895.62) |
DNA isolation
The isolation kit (QiAamp DNA Blood Mini kit, Syngen Biotech, Wroclaw, Poland) was used for DNA isolation from peripheral blood lymphocytes according to the manufacturer’s instructions.
VEGF genotyping
All patients were genotyped by polymerase chain reaction (PCR) for the verification of addition or loss of 18-bp at -2549 -2567 position in the promoter of VEGF gene – the protocol by Lachheb et al. [15] was used. First, the concentration of isolated DNA and its purity were identified using a spectrophotometer (NanoDrop, Thermo Fisher Scientific). In the next step, the PCR mixture was prepared (total volume 25 µl) – it contained 100 ng of genomic DNA, 1× Taq Buffer, 0.5 mmol/l of nucleotide, 3 pmol of suitable starter, and 0.5 U of Taq-DNA polymerase (Taq DNA Polimeraza E2500-02 – 5000u, EURx); the final concentration of MgCl2 was up to 4 mmol/l. The PCR comprised an initial denaturation step (95ºC for 15 min), then 35 cycles (95ºC for 30 s), primer annealing (54ºC for 30 s and 72ºC for 30 s), and the final extension step (72ºC for 10 min). The primers were as follows: forward 5’-CCTGGAGCGTTTTGGTTAAA-3’ and reverse 5’-ATATAGGAAGCAGCTGGAA-3’ (DNA primers, Polgen). Then, the PCR products underwent electrophoresis in agarose gel stained with ethidium bromide. DNA in the form of strips was visible by fluorescence under a UV light transilluminator. The obtained fragment sizes were 216 bp (18-bp deletion) and 234 bp (18-bp insertion). After genotyping, all participants were divided into two cohorts: del (deletion: no mutation – genetic variant del/del; asthmatics vs control: 61 vs 22 participants) and ins (insertion: mutation – genetic variants ins/ins or ins/del; asthmatics vs control: 21 vs 18 participants).
Results
To determine the expression of surface markers CD69 and CD11b, peripheral blood neutrophils collected from asthmatics and controls were incubated in:
1. Medium (negative control)
Freshly isolated neutrophils displayed CD69 and CD11b but culture in medium alone had a little effect on their expression. The median specific fluorescence of active CD69+ and CD11b+ neutrophils was at a comparable level in all tested participants and this difference was not statistically significant (p > 0.05). Besides, genetic variants del/ins at -2549 -2567 position in the promoter of the VEGF gene did not have an influence on active CD69+ and CD11b+ neutrophils (p > 0.05) either in asthmatics or in controls.
2. fMLP (positive control)
Neutrophils incubation in fMLP intensified expression of CD69 and CD11b – the stimulation index was raised. The median specific fluorescence of active CD69+ and CD11b+ neutrophils was at a similar level in asthmatics and controls and this difference was not statistically significant (p > 0.05). Additionally, in asthmatics with ins genotype, the level of active CD69+ and CD11b+ neutrophils was higher than in controls with ins genotype and these results were on the edge of statistical significance (p = 0.05 and p = 0.07, respectively).
3. VEGF (experimentally established concentration – 250 ng/ml and 500 ng/ml)
To explore whether CD69 and CD11b expression could be induced by different VEGF concentrations, neutrophils were incubated with this cytokine for 30 min. There was a dose-dependent induction of these markers’ expression by VEGF – stimulation index was increased for concentration 250 ng/ml and even more for concentration 500 ng/ml. The median specific fluorescence of active CD69+ and CD11b+ neutrophils was at a similar level in asthmatics and controls and this difference was not statistically significant (p > 0.05). Moreover, this difference remained statistically insignificant taking into account the division into del and ins after genotyping among examined groups.
Discussion
The characteristic issues of inflammation observed in asthma includes activated eosinophils, mast cells, increased numbers of natural killer T cells (NKT), T helper lymphocytes (Th2 and Th17) and neutrophils. Each of these cell populations is able to release inflammatory mediators, contributing to symptoms, remodelling process or enhancing resistance to treatment with steroids. Over one hundred mediators are recognized to be involved in the complex airway inflammatory response in asthmatics. The clinical value of measurement of activated cells combining with other markers of airway inflammation may lead to more accurate assessments of the disease stage, clinical phenotypes, optimize diagnosis and treatment [16, 17]. Moreover, possible asthma biomarkers taken from peripheral blood are easy to obtain, the procedure itself is less invasive than bronchoalveolar lavage (BAL) or sputum induction and dynamic recruitment of activated immune cells from the peripheral blood can be used as an indirect readout of the disease state [18].
Different studies have shown that cells, such as granulocytes, respond to inflammatory signals by up-regulating activation markers in chronic inflammatory conditions, e.g. asthma. Some of these markers, including CD69 and CD11b, are typically found in granules shortly after cell activation with inflammatory mediators then fuse with the plasma membrane where might be detected several hours after stimulation [19–21]. What is more, Fortunati et al. [22] compared the presence of markers on blood cells and tissue cells obtained from sputum and BAL and revealed that cells homing to the tissue under homeostatic conditions exhibit the same phenotype. In our previous study we have shown that an increased VEGF serum concentration is characteristic for patients with asthma, especially those with irreversible bronchoconstriction [23]. In other papers [24, 25], matters of basophils VEGF-activation and the potential contribution of VEGF gene polymorphism to the spontaneous increase of eosinophils activity (priming) in patients with asthma were suggested. Also, the genetic variant such as the del18 genotype in the promoter region of the VEGF gene was demonstrated as a possible factor connected with presence of and the risk of irreversible bronchoconstriction in asthmatics [26]. Interestingly, the above might indicate the influence of VEGF and its genetic region on different cell activation state in asthmatics.
In the current study, we found that peripheral blood neutrophils from patients with asthma express CD69 and CD11b on the surface. Obtained data indicate that stimulation of donor neutrophils with increasing concentrations of VEGF enhances the activity of these cells, while no statistically significant differences were noted between the expression of these markers. Also, different genetic variants in the VEGF-promoter region were not relevant to presence of CD69 and CD11b markers on the neutrophils membrane in the examined groups. Considering this context, it is very likely that VEGF is not a meaningful factor affecting neutrophil activation in asthmatics and CD69 and CD11b might be more valuable markers of an increment associated with asthma exacerbation, cell adhesion and transmigration through venular walls. On this basis, future cellular and biomarker evaluation of patients with obstructive airway diseases might be important in the everyday clinic practice [5, 27, 28]. Moreover, we also observed a slightly extend in CD69 and CD11b expression on fMLP stimulated neutrophils in asthmatics with the ins genotype in the promoter region of the VEGF gene. It can be presumed the potential contribution of the VEGF gene polymorphism to neutrophils activity (priming and stand-by) and because of that cells’ response to various unspecific agents such as fMLF/fMLP released during intercurrent bacterial infection. Peripheral blood neutrophils being in different activation states may circulate to the airway endothelium leading to the process of airway inflammation, narrowing and remodelling. In the ongoing neutrophilic inflammatory process in asthmatics, secondary causes e.g. high-dose corticosteroid therapy, exposure to environmental pollution or cigarette smoke should also be considered [29]. Suggesting other signalling pathways, some limitations of our study may include the spectrum of membrane markers chosen for the flow cytometry protocol, VEGF concentrations selected experimentally, asthmatics not divided according to criteria based on the predominance of eosinophils or neutrophils and quite a small number of participants tested in terms of genetic research. Accordingly, it would be valuable to study expression a phenotyping panel with other neutrophil receptors (e.g. CD45, CD14, CD177) and another stimulus (e.g. cytokines or chemokines) on a greater sample in patients with the initial phase, during progression or exacerbation of asthma. Moreover, we agree that our work needs further examinations in different groups including eosinophil or not eosinophil subjects.
Conclusions
Various immune cell types and cytokines are identified as important for asthma endotype definition. In conclusion, we present our findings to demonstrate that VEGF might insignificantly activate neutrophils in asthmatics. In addition, the modulated expression of CD69 and CD11b on peripheral neutrophils may be only suggested that is not related to potential contribution of the VEGF gene polymorphism in the asthmatic group of patients. An additional issue at stake is that peripheral neutrophils might be heterogeneous populations both with respect to the state of maturity and activation. Notwithstanding these handicaps, further elucidation of the neutrophilic inflammation and mechanisms underlying various signalling pathways and their interconnections may help to develop better targets of pharmacotherapy in asthma as an inflammatory disease.