Introduction
Psoriasis is one of the most common inflammatory skin diseases. It is a chronic, recurrent, non-infectious genetic dermatosis that affects 2–3% of the population [1–7] irrespective of sex [8, 9]. It may appear at any age, but most commonly manifests before 40 years of age [10–12]. Psoriasis is typified by accelerated epidermal proliferation and incomplete keratinisation (parakeratosis) of keratinocytes. Several varieties of this seemingly uniform disease can be identified depending on the type of skin lesion and the course of the disease. The most common form is plaque psoriasis, which accounts for about 90% of all cases [13–16]. Clinically, it manifests in the presence of vivid red papules covered with adherent silvery-white scales, which merge into usually symmetrical, well-demarcated plaques. The skin lesions are usually located on the elbows, knees, lower back and scalp, but the extent of skin involvement is variable, ranging from a few localized plaques to erythrodermia [13, 17]. The diagnosis of psoriasis is usually clinical, based on history and physical examination, but it may also be confirmed by histopathological examination.
The pathogenesis of psoriasis is influenced by various genetic, epigenetic [17–23] and environmental factors, as well as immunological disorders. Exposure to UV radiation, infections, injuries, medications, smoking, alcohol abuse, stress and diet may not only provoke the onset of lesions, but also affect the course and severity of the disease [21, 22, 24–28]. Recent studies indicate that a major role in the initiation, maintenance and recurrence of skin lesions is played by T lymphocytes and pro-inflammatory cytokines such as interleukins (IL) IL-12, IL-17, IL-22, IL-23, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) [29–36]; however, important roles may also be played by IL-20 and IL-8 [37–39]. The IL-20 subfamily plays a significant role in the immunity of the epidermis because it not only affects the growth and differentiation of keratinocytes but also influences the production of a number of chemokines, proteases, defensins and growth factors. IL-8 also exerts a potent chemoattractive effect on neutrophils, which aids in their recruitment and thus contributes to the erythema observed in psoriasis; however, IL-8 is believed to exert its influence on the course of psoriasis by various other routes. Despite a wealth of research, the exact course of the pathogenic cascade and the precise contributions of each mediator to the pathogenesis of psoriasis remain unclear.
Interleukin 20
IL-20 is a proinflammatory member [40, 41] of the IL-20 subfamily, along with IL-19, IL-22, IL-24 and IL-26 [40, 42–46]. As this subfamily belongs to the larger IL-10 family, IL-20 is one of the homologues of IL-10. It is produced by keratinocytes, fibroblasts, stimulated monocytes, granulocytes and dendritic cells [47–53]. Its production is mainly stimulated by IL-22, and to a lesser extent by IL-17A and TNF-α, while IFN-γ and IL-20 itself appear to have no influence [48, 51, 54, 55]. This relationship suggests that IL-20, like other tissue cell mediators, is induced by T-cell mediators.
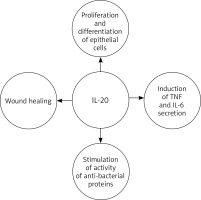
IL-20 plays a role in epidermal function by regulating keratinocyte proliferation and differentiation and wound healing [38, 42, 43, 56–58]. It is believed that in the later effector phase of psoriasis pathogenesis, IL-20 inhibits keratinocyte final differentiation and stimulates neutrophil chemokine production in epidermal cells [51, 52, 58]. It also acts as an autocrine and paracrine growth factor for keratinocytes, and stimulates them to secrete TNF and IL-6 [59]. Thus, it can be assumed that IL-20 acts as a secondary mediator, reinforcing the pathogenic changes in the epidermis caused by T-cell mediators, which in turn sustain the characteristic skin changes observed in psoriasis. In addition to inhibiting cellular differentiation, IL-20, along with other molecules [60, 61], stimulates the activity of anti-bacterial proteins that are also overexpressed in psoriasis [38, 51]. This is reflected in the clinical picture of the disease: despite the disturbance of the integrity of the epidermis, skin infections are quite rare in cases of psoriasis (Figure 1).
In signalling, IL-20 requires a heterodimeric receptor consisting of the β subunit of the IL-20 receptor (IL-20R2), which is indispensable, together with either the α subunit of the IL-20 receptor (IL-20R1) or the α1 subunit of the IL-22 receptor (IL-22R1). As such, it signals through two alternative receptor complexes: IL-20R1/IL-20R2 (type I) and IL-22R1/IL-20R2 (type II) [43, 53, 62–65]. Despite having two receptor complexes, some data suggest that IL-20 can cause psoriasis-like lesions mainly through IL-22R1/IL-20R2 rather than IL-20R1/IL-20R2 [50, 58, 66]. These receptors are expressed mostly in the skin but also in lungs, ovaries, testis, kidney and other tissues, suggesting additional target tissues for IL-20 action [38, 43, 47, 50, 67, 68]. It is worth mentioning that complete receptor complexes are not found on immune cells: only IL-20R2 expression was found in some peripheral blood mononuclear cell (PBMC)-derived cells [38, 47, 50, 69, 70]. Hence, it can be assumed that most of the effects of IL-20 on keratinocytes are mediated by the IL-22R1/IL-20R2 receptor. It is also important to note that receptor complexes for IL-20 are also used by other members of the IL-10 family; for example, the IL-20R1/IL-20R2 complex binds not only IL-20 but also IL-19 and IL-24, and the IL-22R1/IL-20R2 complex also binds IL-24 [50, 53, 67, 71].
Interleukin 20 in tissue
Upregulation of IL-20 mRNA, IL-20 proteins and IL-20 receptors are known to be upregulated in psoriatic-lesional skin, indicating that IL-20 plays a role in the mechanisms of psoriasis.
IL-20 has been found to be overexpressed in lesional psoriatic plaques in many studies. Rømer et al. [72] observed focal expression of IL-20 mRNA in suprapapillary epidermis in lesional skin but not in skin without psoriatic lesions. Similarly, psoriatic plaques have demonstrated increased expression of IL-20 compared to non-lesional and/or healthy skin in other studies [37, 50, 54, 66, 73, 74]. A highly elevated IL-20 mRNA level, which positively correlates mainly with the level of IL-22 but also IL-17A and TNF-α, also has been reported in lesional psoriatic skin [51]. However, as chronic psoriatic plaques have been studied, it is impossible to determine whether IL-20 is a trigger of the disease or just a factor that maintains the occurrence of lesions. The distinct localization of IL-20 mRNA expression at the top of papillae and its absence in monocytes/macrophages, melanocytes, Langerhans dendritic cells, endothelial cells or lymphocytes suggests that the main sources of IL-20 mRNA in the psoriatic lesions are keratinocytes [72].
Upregulated IL-20R1 and IL-20R2 mRNA expression has also been reported in the epidermis of psoriatic skin, as noted by Blumberg et al. [43]. Similarly, Rømer et al. [72] note the expression of mRNA for the receptor chains of IL-20R1, IL-20R2 and IL-22R1 in the psoriatic epidermal layer; while these patterns have also been detected in non-lesional psoriatic skin, their expression was not as strong as in plaques. This may be due to the sampling protocol, or some unknown condition of non-lesional skin adjacent to psoriatic lesions. In contrast, no mRNA expression for these receptors could be detected in the cells of the dermis [72]. Also increased expression of IL-20R1 and IL-20R2 was confirmed in psoriatic epidermis versus healthy individuals by Wei et al. [37], while Wolk et al. reported nearly doubled IL-22R1 expression in psoriatic skin in comparison to controls [58]. Such overexpression of IL-20 receptors in the epidermis is likely to alter the relationships and interactions between immune cells, endothelial cells and keratinocytes, with consequent dysregulation of keratinocyte proliferation and their differentiation.
However, contradictory results have also been obtained. Diminished mRNA levels of subunits IL-20R1 and IL-20R2 have been noted in psoriatic skin compared to nonlesional and normal skin [54, 66], with comparable expression of IL-22R1 mRNA observed in lesional and nonlesional psoriatic skin [66], which may suggest that the IL-22R1/IL-20R2 complex plays a greater role in the pathogenesis of psoriasis than the IL-20R1/IL-20R2 complex. This apparent contradiction may be caused by differences in research methods, e.g. quantitative RT-PCR vs. in situ hybridization and the theory that differential expression of the cytokine itself is more important in mediating the action than the receptors. In addition, researchers in Denmark identified a negative correlation between the mRNA expression of IL-20 and its receptors (IL-20R1 and IL-20R2) [66]; indeed, higher expression of IL-20 would entail a compensatory reduction in the number of its receptors. However, another study [38] found that while primary human keratinocytes express the IL-20 receptors IL-20R1, IL-20R2 and IL-22R1, all three proteins were expressed in the epidermis; it is therefore unclear whether IL-20 preferentially chooses one of the receptor pairs (IL-20R1/IL-20R2 or IL-22R1/IL-20R2). Even so, it has also been found that IL-20R1 and IL-22R1 may play complementary roles in IL-20 signalling in human keratinocytes [38, 67]. It is also important to note that no surface expression of IL-20 subfamily receptors has been recorded on T cells, B cells, NK cells or monocytes, but it has been identified on keratinocytes [38, 47, 50, 69, 70]. As it is mainly produced by immune cells, with its receptors expressed on keratinocytes, IL-20 may act as a messenger between the two cell types.
These findings support the current view that IL-20 and its receptor complex play a direct or indirect role in the function of the epidermis in psoriasis by influencing the proliferation and differentiation of keratinocytes. However, whether IL-20 plays a role in the initiation of psoriasis, or only in its maintenance, remains to be investigated in future studies.
Interleukin 20 in serum
Few studies have examined the serum level of IL-20. However, Michalak-Stoma et al. [75] reported that psoriatic patients demonstrate significantly higher IL-20 serum concentration than controls, and a positive correlation was observed between IL-20 blood levels and psoriasis area and severity index (PASI). Similarly, in another study [51] psoriatic patients demonstrated increased serum levels of IL-20 protein, which positively correlated with PASI compared to healthy participants, indicating that IL-20 level in blood correlates with disease severity and can serve as a marker of psoriasis activity.
But there are also contradictory data. Wei et al. [37] found a markedly lower level of IL-20 in the serum of psoriatic patients compared to healthy controls; in addition, no correlation was observed between serum IL-20 levels and PASI score. The significantly lower IL-20 level may be due to greater binding to the higher concentration of IL-20 receptors, and/or the accelerated uptake of IL-20 by keratinocytes in psoriasis patients. It is also thought that the major role in psoriasis pathogenesis is played by Th1 lymphocytes; this would result in a lower concentration of Th2 cytokines such as IL-2, IL-4 and IL-10 in patients with psoriasis and hence a disparity of CD4+ T-cell differentiation and a possible reduction of serum IL-20 levels.
Interleukin 20 and treatment
Two studies found that treatment with oral immunosuppressant drugs such as cyclosporin A and topical calcipotriol (a synthetic analogue of 1,25-dihydroxyvitamin D3) led to a decrease, or even disappearance, of IL-20 mRNA overexpression in psoriatic plaques, resulting in clinical and histopathological improvement [66, 72]; both drugs affect the cytokine network in psoriatic plaques by controlling one or more of the pro-inflammatory factors. Cyclosporin, as an inhibitor of calcineurin, prevents T-cell activation, which consequently causes inhibition of IL-2 production in Th1 lymphocytes [76]; calcipotriol lowers IL-8 and increases IL-10 within the lesions but also has a direct effect on keratinocyte proliferation and differentiation [77]. Cyclosporin is a more potent systemic drug, and caused a greater decrease than the topical calcipotriol. However, cyclosporin or calcipotriol treatment had no statistically significant effect on IL-20R1, IL-20R2 or IL-22R1 mRNA expression [66, 72]. Similarly, Wolk et al. [51] reported significantly decreased expression of cutaneous IL-20 mRNA in treated patients at the end of phototherapy (PUVA and UVB-NB).
Infliximab (anti-TNF-α chimeric monoclonal antibody) treatment was found to reduce IL-20 mRNA expression in the skin of patients suffering from psoriasis [73]. The same effect was obtained after therapy with alefacept: a fully human fusion protein composed of fragments of the lymphocyte function-associated antigen (LFA)-3 molecule and human immunoglobulin G that targets CD2 on T cells; treatment is known to reduce T lymphocyte numbers in psoriatic plaques [78, 79]. Successful alefacept treatment resulted in a marked decrease in IL-20 expression in psoriatic lesional skin [54, 80]. A positive correlation has also been noted between IL-20 expression and PASI score [80], as well as another objective parameters of disease activity, i.e. the response score (which combined epidermal thickness, K16 mRNA, and K16 staining) [54]. These data, however, did not apply to non-responders, in whom the level of IL-20 remained unchanged despite the therapy [54]. Ustekinumab, a human monoclonal IgG1κ antibody that binds to the p40 protein subunit shared by IL-12 and IL-23, also caused a decrease in the level of IL-20 mRNA expression in psoriatic skin; the level was similar to that of non-lesional skin after 112 days of treatment. However, as in the case of alefacept, this effect was visible only in the responder group [74]. Etanercept, a soluble recombinant p75 Fc receptor protein binding to TNF-α, also had the same effect in lowering IL-20 mRNA levels [81]. In contrast, therapy with recombinant human IL-4 did not result in a reduction of IL-20 or both IL-20 receptor chains’ mRNA levels in psoriatic patients, although a clinical improvement was noted [82].
Treatment targeting IL-20 receptors is a promising therapeutic option in psoriasis, particularly that targeting IL-22R1. This type of therapy should prevent any disease consequences associated with IL-22, i.e. via receptor complex IL-22R1/IL-10R2 [83–85], and IL-20, via receptor complex IL-22R1/IL-20R2. This is supported by the fact that IL-22 can stimulate IL-20 production in epidermis and keratinocytes [51] and that healthy people naturally have more IL-22R1 receptors than IL-20R1, with this difference being even greater under the influence of TNF-α and IFN-γ, as is the case in psoriasis [50]. In addition, as IL-20 receptor complexes are not expressed on immune cells, any therapy targeting the IL-20 receptors would also avoid any immunosuppressive effects [38, 47, 50, 69, 70].
Stopping the action of IL-22 and IL-20 proved effective in a mouse model of psoriasis-like skin inflammation and in a human xenograft transplantation model. Blocking these cytokines prevented the development of psoriatic lesions, indicating their critical role in the induction of psoriasis [40, 41, 86]. IL-20 also appears to play a role in the maintenance of psoriatic lesions: these lesions were found to subside when anti-IL-20 antibodies were administered to grafted mice with lesional psoriatic skin [40]. This suggest that IL-20 may be a potential target in psoriatic treatment. Unfortunately, treatment with fully human recombinant monoclonal antibodies neutralizing IL-20 did not yield any apparent improvement of PASI scores in people suffering from psoriasis [87]. However, it should be emphasized that the aim of this trial was to investigate the tolerability and safety of an anti-IL-20 drug; as such, the lack of effectiveness of the therapy may be related to insufficient doses or excessive periods between drug dosing. Nevertheless, the overall picture may suggest that more than one IL-20 subfamily member should be inhibited at any one time to obtain clinical improvement, as each one (IL-19, IL-20, IL-22 and IL-24) induces hyperplasia and thickening of the epidermis, independently of the others [87]. It has also been shown that the administration of recombinant human IL-20 to transplanted psoriatic plaques did not exacerbate the disease symptoms and was not enough to induce lesions in nonlesional psoriatic skin. Interestingly, if leukocytes (PBMC) were added to IL-20, psoriatic lesions were induced in nonlesional skin grafts, which suggests that several factors may be needed to cause psoriasis, not only IL-20 [41].
In summary, it is well known to clinicians that variable responses are obtained by topical drugs and systemic treatments, including biological therapy. Even so, therapeutic intervention targeting IL-20 and/or its receptors seems to be a promising therapeutic direction in psoriasis, and a combined anti-IL-22/IL-20 approach might be even better.
Interleukin 8
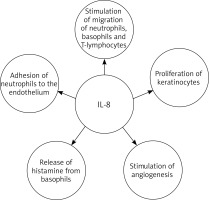
Interleukin 8 (IL-8), also known as chemokine ligand 8 (CXCL8) or neutrophil activating protein-1 (NAP-1), is a pro-inflammatory chemotactic cytokine, stimulating the migration of neutrophils [88–91], basophils [92] and T lymphocytes [93]. Moreover IL-8 causes adhesion of neutrophils to the endothelium and indirectly induces their degranulation, as well as histamine release from basophils and stimulation of angiogenesis [59, 94–98]. It also has a proliferative effect on keratinocytes [99, 100] Hence, IL-8 may be one of the factors responsible for inducing excessive epidermal growth in psoriasis (Figure 2). Moreover IL-8 may be one of the inducers of neutrophil extracellular traps (NETs). NETs may contribute to the release of IL-17 and may lead to the secretion of cathelicidin LL-37 and DNA complexes, which in turn may activate the secretion of interferon-α by dendritic cells. Additionally there was a positive correlation between NET formation and psoriasis severity. These data suggest that NETs may therefore play a role in the pathogenesis of psoriasis [101, 102].
IL-8 is mainly produced by monocytes/macrophages, T lymphocytes, neutrophils, fibroblasts, endothelial cells and keratinocytes [91, 103, 104], but this cytokine is released by neutrophils under certain activation conditions [105–108]. Its action is greatly enhanced by IL-1 and TNF-α [109].
To induce a biological effect, IL-8 must bind to specific functional surface receptors, particularly the G-protein-coupled receptor family members CXCR1 (formerly IL-8RA) and CXCR2 (formerly IL-8RB) [110, 111]. CXCR1 is highly specific for IL-8 and granulocyte chemotactic protein-2, while CXCR2 besides IL-8 also binds to other molecules [112]. The receptors for IL-8 are located on numerous cells, including neutrophils, T lymphocytes, monocytes/macrophages, endothelial cells and keratinocytes [113].
Interleukin 8 in tissue
IL-8 mRNA was found to be elevated in skin biopsies taken from psoriasis plaques compared with samples from healthy volunteers [91, 114–118]. Similarly, lesional tissue from psoriatic patients was found to demonstrate increased IL-8 mRNA expression compared with uninvolved biopsy specimens [73, 74, 116, 118–121]. Various other studies have noted upregulation of IL-8 in psoriatic tissue [122, 123]. Sticherling et al. [39] also observed elevated amounts of IL-8 in psoriatic lesional skin and noted a positive correlation with disease severity. IL-8 mRNA overexpression has also been noted in suprabasal keratinocytes of involved skin [91, 114, 120, 121], although earlier data indicated restriction to basal keratinocytes [124]. The upregulation of IL-8 in epidermal cells may be caused by the inhibition of IL-8 downregulation, and/or an enhanced response of keratinocytes to IL-8 inducing factors. Increased expression of this cytokine in the skin is associated with the accumulation and formation of neutrophil infiltration, which in turn is connected with the presence of inflammation and treatment-resistant psoriasis [125]. Moreover, this overexpression of IL-8 helps to maintain this inflammatory infiltrate through positive feedback.
A marked increase in the epidermal expression of IL-8R has been noted in lesions in psoriasis compared to both normal and/or uninvolved skin [116, 126]. Beljaards et al. [126] reported that keratinocytes expressed the IL-8 type B receptor while neutrophils expressed type A.
The expression of IL-8 and its receptor are significantly increased in psoriatic epidermis. Thus, it appears that IL-8 may be considered a useful marker for monitoring disease severity and therapeutic effects, and IL-8R may be a molecular target for anti-psoriatic drugs.
Interleukin 8 in serum
While significantly higher levels of IL-8 have been noted in the serum of patients with psoriasis compared to healthy controls [97, 98, 127–130], some studies have found no correlation between IL-8 level and PASI score [39, 98, 127, 130]. As such, serum IL-8 level does not appear to be an objective indicator of the clinical severity and activity of the disease. In addition, this value did not correlate with age or gender [127].
Other scientists have made different observations. Serum IL-8 levels also have been found to be elevated in psoriasis patients compared to people without the disease, but to positively correlate with PASI [131] or degree of erythema [97]. This suggests that IL-8 could be regarded as a predictor of psoriasis activity. Also serum IL-8 level was found to positively correlate with age [98].
However, it is not possible to determine the origin of cytokines in serum, and their excess may result from overproduction, excessive deposition in tissues or from insufficient elimination. Conversely, lowered levels may result not only from decreased production, but also from inactivation or binding. It is worth mentioning that the results may also differ depending on the method of cytokine determination, which can also be influenced by the presence of other chronic diseases and medications [132]. It is also not known whether abnormal levels of IL-8 in the blood are the cause or a result of the development or maintenance of psoriasis. Therefore, it is impossible to conclusively state that IL-8 serum levels can serve as diagnostic or prognostic criteria in psoriasis. Further studies are needed to establish the exact role of serum cytokines, such as IL-8, in the aetiopathogenesis of psoriasis.
However, no data currently suggest that psoriasis patients demonstrate a decreased level of IL-8 in the serum in comparison with the healthy population.
Interleukin 8 and treatment
Most studies suggest that conventional and biological treatment affects both blood and tissue levels of IL-8. Kang et al. [77] found a lower IL-8 level in the lesional skin of a group of psoriasis patients treated with calcipotriene in comparison to those who received placebo (only vehicle). Similar results were obtained using topical calcitriol: after eight-week treatment, the immunoreactivity for IL-8 in the epidermis changed to a pattern characteristic of non-lesional psoriatic or normal human skin [133]. Elsewhere, oral cyclosporin therapy significantly lowered IL-8 mRNA in skin biopsies taken from psoriasis plaques [117]. Interestingly, in these 2 cases, reductions in IL-8 level occurred prior to clinical improvement, indicating that IL-8 alterations precede psoriatic lesion regression. This is in agreement with Lemster et al. [134], who reported the downregulation of IL-8 mRNA in psoriasis lesions in patients on oral tacrolimus treatment after effective therapy. However, no reduction of IL-8R was observed in the same post-treatment biopsies, which is in contrast to an in vitro study in which tacrolimus was found to inhibit expression of the IL-8 receptor in human keratinocytes as compared with untreated cells [116]. A similar reduction in IL-8 mRNA expression was induced by ustekinumab, infliximab and etanercept in the responder tissue of lesional psoriatic skin compared with baseline [73, 74, 81, 119, 135]. This decrease also positively correlated with clinical improvement [73, 74]. Recombinant human IL-4 therapy also led to a reduction of IL-8 mRNA in psoriatic lesions compared to the state before treatment [82].
Also Lemster et al. [134] reported a decline of circulating IL-8 level in blood together with improved clinical condition in response to systemic tacrolimus treatment. Remission, reflected as a reduction in PASI score, was accompanied by a drop in IL-8 level, while exacerbation was associated with an increase. This is consistent with the study by Zalewska et al. [131], in which three-week anti-psoriatic treatment (anthralin with or without UVB-NB phototherapy, tar with UVB-NB phototherapy, methotrexate) lowered the level of IL-8 in the serum of patients, but not to the level observed in healthy volunteers. However, Sticherling et al. [39] reported an almost unchanging level of IL-8 in serum during effective anti-psoriatic therapy (topical steroids under occlusion or dithranol with PUVA or UVB treatment). Also no decrease in blood IL-8 level was detected after infliximab treatment, despite clinical improvement of psoriatic lesions [73].
A topical IL-8 inhibitor (Abcream) was found to be effective against psoriasis, manifested by a decrease in PASI [136]. Based on the findings, the medicine was approved for psoriasis therapy in China, but not elsewhere. The use of systemic biological agents has also been studied. Theoretically, IL-8 monoclonal antibodies should reduce neutrophil influx and inhibit angiogenesis. However, a fully human monoclonal antibody targeting IL-8 (ABX-IL8) unfortunately showed insufficient effectiveness in lowering PASI compared to placebo. Therefore, further research on anti-IL-8 has been discontinued [136].
Another study examined whether treatment may also affect IL-8 receptor expression. Topical betamethasone dipropionate or topical calcitriol resulted in a decrease in IL-8R type B on keratinocytes and histological improvement [126].
The discrepancies observed between the studies may be due to the different mechanisms used by the studied drugs and their different efficacies. In addition, they could be associated with their different effects on IL-8, both systemically and locally at the site of inflammation.
Conclusions
The exact course of the pathogenetic cascade and the precise contribution of each mediator in the pathogenesis of psoriasis are still matters of debate. Nevertheless, it seems that IL-20 and IL-8 may play important roles.
The increased levels of IL-20 and IL-8 and overexpression of IL-20R and IL-8R in psoriatic skin may act in concert to induce the main symptoms of psoriasis: leukocyte infiltration and epidermal hyperproliferation. These cytokines, especially IL-20, can be considered as useful markers for monitoring patients with psoriasis. They may also be suggested as potential targets in therapy, as blocking their activities or their receptors may provide benefits and delay the progression of psoriasis. However, due to the different therapeutic response among patients, it is necessary to search for new treatment options.
Nevertheless, more research is needed to better understand the role these cytokines play in the development and maintenance of psoriatic lesions, to identify molecular mechanisms regulating IL-20 and IL-8 expression, and to understand how IL-20 and IL-8 interact with other cytokines known to influence the pathology of psoriasis.