Purpose
Following initial local and regional treatment of breast cancer, nearly 30% of patients will experience a recurrence of the disease [1]. Recurrences can be classified as local recurrence confined to the chest wall, regional relapse, including ipsilateral axillary, supraclavicular, or internal mammary nodal (IMN) metastasis, and distant metastases, especially to bones and viscera. Although IMN recurrence along with other metastases is common, isolated IMN recurrence is rare [2].
A meta-analysis has shown that loco-regional radiotherapy, including regional nodal irradiation, significantly reduces the risk of loco-regional recurrence, breast cancer (BC) mortality, and overall mortality in node-positive patients [3]. However, the inclusion of IMN chain in regional nodal irradiation is controversial. 15-year results of the EORTC 22922/10925 trial, which included patients with positive axillary nodes or medial/central tumors, showed a significant reduction in BC recurrence and mortality, but no difference in overall survival using irradiation of IMN and medial supraclavicular region [4]. Similarly, a Danish Breast Cancer Group study showed that prophylactic IMN treatment as part of loco-regional radiotherapy in node-positive patients reduced the risk of distant recurrence and death from breast cancer, thereby improving long-term survival. However, in this study, patients with left-sided tumors did not receive IMN radiotherapy because of concerns about cardiac toxicity [5].
Another randomized trial from South Korea did not find significant disease-free survival advantage with IMN irradiation (IMNI), except for patients with medial and centrally located tumors [6]. According to a recent systematic review and meta-analysis, IMNI is associated with a statistically significant improvement in survival in high-risk patients; although, the magnitude of clinical benefits is questionable [7].
These studies support the routine use of IMNI for right-sided medio/central breast tumors. However, its routine use on left-sided tumors, which are associated with increased cardiac toxicity, is still debatable [5].
Oligo-metastatic disease has been described in BC. Given the likelihood of limited spread, it is possible to achieve prolonged survival, and in 2-3% of cases, cure with aggressive metastasis-directed therapy [8]. Stereotactic ablative radiotherapy (SABR) is the preferred method of metastatic-directed therapy, and is used specifically for oligo-metastases from BC to the brain, liver, lung, and spine, with excellent local control [9]. Oligo-metastases to lymph nodes are less well-studied in BC; however, there is evidence suggesting using SABR with excellent local control, especially in supraclavicular lymph nodes [10].
There is no standard salvage strategy for IMN recurrence. The present case report described the use of image-guided interstitial brachytherapy (brachy-ablation) as a promising therapeutic option for internal mammary node recurrence in triple-negative BC patients. Interstitial brachytherapy is a highly precise and minimally invasive radiation therapy technique that utilizes percutaneous needles or catheters to deliver high doses of radiation to a specific area, while limiting exposure to surrounding healthy tissue.
Case presentation
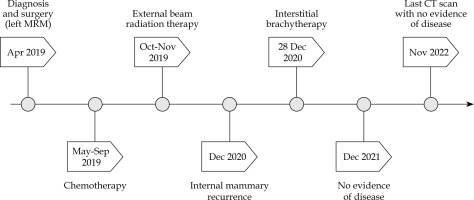
In April 2019, a 68-year-old woman presented with a 3 cm tumor at the 8 o’clock position in the lower inner quadrant of her left breast with a single mobile axillary node. A fine needle aspiration was done, which showed infiltrating ductal carcinoma. The patient subsequently underwent a modified radical mastectomy surgery. Histopathology report showed infiltrating ductal carcinoma grade 2 and a single positive axillary node without extra-capsular extension, with a pathological stage of T2N1M0 and negative for ER/PR and Her2/neu. The patient underwent chemotherapy and radiotherapy consisting of 3 cycles of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC), followed by 3 cycles of paclitaxel every three weeks. Subsequently, she received post-mastectomy radio-therapy to the chest wall, and supraclavicular fossa at a dose of 50 Gy in 25 fractions over 5 weeks with a 6 MV photon beam and a 1 cm bolus to the chest wall every alternate day. Field arrangement for the chest wall was the standard tangential pair, and no attempt was made to deliberately cover the internal mammary area. The supraclavicular area was treated with a direct anterior beam.
In December 2020, a single oval-shaped internal mammary node recurrence was reported during a routine follow-up CT scan. The enlarged node measured 14 × 11 mm in size, and was located in the extra-pleural space in the parasternal position, posterior to the left second costal cartilage, and just lateral to the left internal mammary vessels. A fine needle aspiration of the lump was performed from the node, which revealed the presence of metastatic carcinoma. A staging CT scan of the chest, abdomen, and pelvis as well as a bone scan were performed, with no other metastatic lesions detected. The measured CA15-3 level was 24 U/ml. Positron emission tomography/computed tomography (PET-CT) could not be performed due to unavailability.
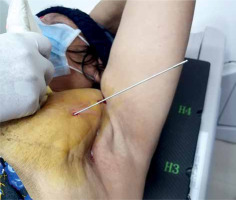
Considering the patient’s desire to have only local therapy and to avoid intravenous systemic chemotherapy, we opted to treat her with interstitial brachytherapy. The procedure was thoroughly explained to the patient, and informed consent was obtained. The procedure was carried out in a CT-scan room equipped with both a CT scan (Toshiba Alexion, Japan) and ultrasonography (Samsung HS40, Korea). The lesion was localized, and the patient underwent the procedure with local anesthesia only using lignocaine infiltrated into the overlying soft tissue. Under ultrasound guidance using a high-frequency linear transducer and Doppler mode, a single brachytherapy needle was safely inserted percutaneously into the internal mammary lymph node (Figure 1). This approach facilitated visualization of the internal mammary vessels separately from the lymph node and minimized the risk of hemorrhage. A 17-gauge, 200 mm in length, CT scan-compatible stainless-steel needle was used, which was advanced beyond the lesion, taking into account 4 mm dead space at the tip. The position of the needle within the lymph node and in relation to adjacent vessels and lung parenchyma was confirmed by a CT scan. The applicator was secured in position using dental putty. No other immobilization device was utilized during the treatment. The resulting 1 mm pitch CT images were then transferred to planning system (BrachyVision version 15, Varian, USA). Gross tumor volume (GTV) and normal structures were delineated, and a treatment plan was designed to deliver a dose of 20 Gy (EQD2 50 Gy, α/β = 10) to the periphery of the lesion (Figures 2A-C, 3). The source was loaded up to 2 cm with a 2 mm step size, and manual optimization was performed to ensure that the prescribed isodose covered 100% of GTV. The GTV, V150% dose, and V200% dose were determined to be 1.4 cc, 1.20 cc, and 0.94 cc, respectively. Doses to organs at risk were measured (Table 1). Pulmonary tissue volume receiving more than 20 Gy EQD2 (α/β = 3) was 5.61 cc.
Fig. 2
Representative CT scans (A) coronal, (B) sagittal, and (C) transverse from the treatment planning system with an isodose line covering the target and surrounding organs at risk. Pink line indicates gross tumor volume, green line denotes D100%, and blue line shows D50%
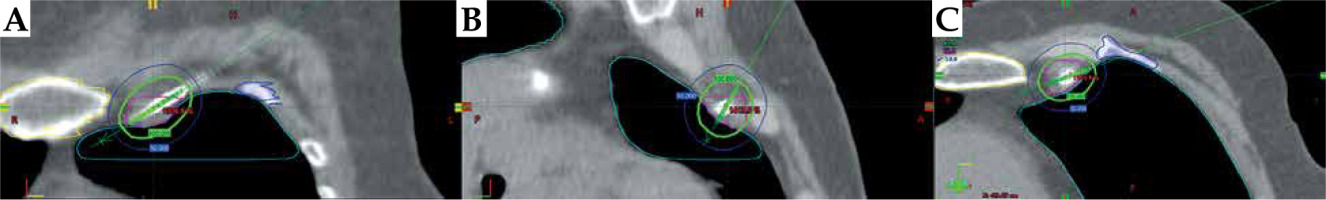
Fig. 3
Planning CT scan of the patient showing the brachytherapy needle and target volume, indicated by pink line
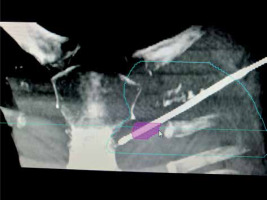
Table 1
Measurement of dose to organ at risk (OAR)
The treatment plan was then transferred to high-dose-rate (HDR) remote afterloader unit with an iridium-192 source (GammaMed iX, USA), and radiation was delivered. Once the radiation delivery was completed, the needle was promptly removed. The radiation delivery time was 6 minutes, and the overall time from the needle insertion to removal was 70 minutes. The patient was admitted overnight for observation, during which she experienced minimal pain controlled with ibuprofen. The patient tolerated the procedure well with no adverse effects, and was discharged the following day after a chest X-ray to check for pneumothorax.
Following brachytherapy, the patient was prescribed oral capecitabine as systemic therapy for one year in adherence with the international consensus guidelines for advanced breast cancer, specifically addressing the recommendation for regional recurrence [11].
Clinical outcome
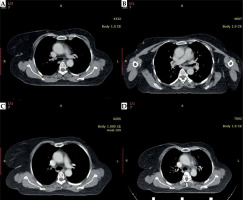
During the follow-up CT imaging in April 2021, an 8.5 x 5.5 mm oval-shaped left internal mammary node was identified, which was significantly reduced in size compared to the pre-treatment scan. Subsequent CT scans were performed in December 2021 and May 2022, which showed a complete response, and her most recent scan done 2 years after the procedure continues to show a complete response (Figure 4A-D). The patient is being closely monitored for any signs of recurrence. There is no long-term adverse effect pertaining to brachytherapy.
Discussion
Earlier findings have revealed that metastases to IMN have been found to occur in the range of 4% to 65% of cases, with a higher likelihood in medially located tumors and those with heavy axillary nodal burden. Possible risk factors associated with IMN metastasis include positive axillary nodes, medial location of the tumor, and age < 35 years [12-15].
The use of regional nodal radiotherapy, including internal mammary region, in breast cancer is still unclear in terms of indications and treatment volume. Its potential benefit for node-positive (N1-2) patients, as recommended by recent meta-analyses, is found to be limited by its toxicities, such as radiation pneumonitis and radiation exposure to the heart [16-18]. Moreover, most recurrences are common in the supraclavicular fossa, followed by the axilla, unlike IMN failure. The increased dose to the lungs and heart is due to the additional radiotherapy portal to cover IMN node [19, 20]. Darby et al. reported that increasing the dose of irradiation to the heart by 1 Gy was associated with an increased risk of ischemic heart disease of approximately 7% [21]. Therefore, the benefit of prophylactic IMN irradiation, especially in low-risk cases with the added risk of lung and cardiac dose, does not seem justified.
Internal mammary nodal recurrence without distant metastases has a more favorable prognosis than patients with distant metastases, although it is very uncommon. Therefore, aggressive local radiotherapy is recommended as a salvage therapy for these patients with IMN recurrence [11, 22].
To date, only a single-case report by Kishi et al. has described the novel use of interstitial brachytherapy in this setting. The authors applied brachytherapy as a boost treatment for the internal mammary node following adjuvant external beam radiation, and used hyaluronic acid as a spacer to decrease the skin dose. In our experience with recurrent cases, we have employed this treatment modality without the use of a spacer, while ensuring that the skin dose remains within safe limit for effective delivery of brachytherapy. The use of a spacer may not be necessary in all cases, and can be individualized based on skin dosimetry [23].
Although SABR may be a potential alternative treatment for internal mammary lymph node metastases, current literature supporting its use is lacking. Moreover, chest wall pain and rib fractures are recognized side effects [24], and the access to SABR is limited in resource-limited countries. It is therefore essential to assess the potential benefits and risks of each treatment modality on a case-by-case basis. This evaluation should take into account individual patient’s factors and available resources to determine the most appropriate treatment option for each patient. Selection of oligo-metastatic patients, especially with low tumor burden, long disease-free interval, and good performance status for aggressive treatment with local ablative therapy, has been shown to improve outcomes. Our paper supports this hypothesis of treating well-selected oligo-metastatic patients with curative intent therapy [25].
PET-CT scanner was unavailable in our case, despite the fact that this imaging modality has significant therapeutic implications in the management of radiation therapy for breast cancer [26].
Conclusions
Interstitial brachytherapy should be considered as a potential treatment option for internal mammary node recurrence in breast cancer. The procedure is safe and well-tolerated, and early results suggest that it is effective for local control. Further studies are needed to determine the long-term efficacy of this treatment modality.