Perioperative goal-directed therapy (PGDT) has been shown to significantly decrease the complications and risk of death, as well as diminish the health care costs in high-risk patients undergoing noncardiac surgery [1–5]. To prevent under- or over-resuscitation and inadequate oxygen delivery, which are all related to worse outcomes, fluids – guided by tests of fluid responsiveness, vasopressors, red blood cells, and in some cases, dobutamine are pre-emptively used [6].
There is a considerable gap between the accumulating evidence on the benefits and the actual adherence to PGDT. Strategies that aim to optimise blood flow are widely adopted in routine care. Cardiac output monitoring has been used in only 10% to 20% of major noncardiac surgery patients during perioperative care and by about one-third of anaesthesiologists in Europe and the United States [7]. Lack of knowledge and availability of monitoring technologies are elements that account for the infrequent use of haemodynamic monitoring. Cost restraints may also play a role in the low adherence to these practices in low-resource settings.
Markers of oxygen delivery/oxygen consumption (DO2/VO2) balance, such as central venous oxygen saturation (ScvO2), and global tissue dysoxia, such as lactate, have been used as the goals during PGDT, but the results have not been consistent. Normalisation of venoarterial carbon dioxide difference [P (v–a) CO2] or CO2 gap has been proposed as a complementary tool or potential therapeutic target by some authors [8, 9]. The CO2 gap is inversely related to the cardiac output, and values > 6 mm Hg suggest low blood flow. The use of the CO2 gap was described as a good marker of blood flow adequacy or microvascular perfusion in critically ill patients under mechanical ventilation [10]. It was reported as a reliable prognostic indicator in patients under mechanical ventilation and in high-risk surgical patients [11, 12]. In septic patients with ScvO2 ≥ 70%, the presence of persistently high CO2 gap has been associated with worse outcomes [13]. We conducted this study to evaluate the ability of haemodynamic therapy, based on the optimisation of the CO2 gap, and individualised fluid administration guided by PPV in reducing the rate of moderate and severe postoperative complications.
METHODS
Study design and participants
This was an explanatory, prospective, historically controlled, before-and-after study that evaluated the effects of a haemodynamic management protocol that was newly implemented and introduced into routine operative care in September 2015 for open major oncological surgeries, in comparison with the effects of conventional management. Patients who were treated between 1 August, 2013 and 31 December, 2017 were enrolled in the study according to the following inclusion criteria: elective open upper gastrointestinal surgeries (gastrectomy or esophagectomy) that were expected to last for more than two hours, family and physician’s agreement on total postoperative support, and postoperative survival expectancy of at least three months. The exclusion criteria were previous haemodynamic instability, presence of infection and cardiac arrhythmias, and emergency surgery. Ethics committee approval was obtained from the local ethical review board (CAAE 63997717.6.0000.5415), and informed consent was waived because of the observational nature of the study (amendment: 2.503.527). This manuscript adheres to the applicable CONSORT guidelines.
The general goals of therapy for both groups (control and intervention) were maintenance of peripheral oxygen saturation (SpO2) > 95%, mean arterial pressure (MAP) > 65 mm Hg, end-tidal CO2 between 35 and 45 mm Hg, and core temperature > 36°C. The Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (P-POSSUM) was used to estimate morbidity and mortality [14].
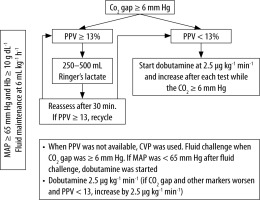
Patients in the intervention group (IG) were managed with a treatment algorithm that was started in September 2015 to December 2017 and targeted first to keep the CO2 gap < 6 mm Hg. PGDT was started if the CO2 gap was ≥ 6 mm Hg, as shown in Figure 1.
After induction of anaesthesia, central venous and arterial catheters were placed for blood sampling and measurements of the MAP and PPV. PPV was calculated during the respiratory cycle as (PPmax – PPmin) / (PPmax + PPmin) / 2 × 100 (Drager Infinity Vista XL, Drager Medical System). Fluid maintenance was kept at 6 mL kg-1 h-1. Blood samples for measurements of haematocrit, electrolytes, arterial and venous blood gases, and serum lactate were obtained within the following time windows: 30 to 60 min (T1), 2 h ±30 min (T2), 4 h ±30 min (T3), and upon intensive care unit (ICU) admission (T4). A bolus of 250–500 mL of lactated Ringer’s solution was given if the PPV was > 13%. Red blood cell transfusion was given if the haemoglobin was < 10 γ dL-1. If the MAP was < 65 mm Hg, noradrenaline was started at a dose of 0.05 μg kg-1 min-1 and was increased as necessary. Thereafter, for persistent CO2 gap ≥ 6 mm Hg and PPV < 13%, dobutamine was started at a dose of 2.5 μg kg-1 min-1 and was titrated up to a maximum of 20 μg kg-1 min-1 to reach the CO2 gap target. Dobutamine was discontinued if the heart rate increased to > 20% of the baseline. The volume of crystalloids, blood products, and use of vasoactive drugs were recorded.
Data from consecutive eligible patients who were operated on by the same team of surgeons and anaesthesiologists before the institution of the new protocol (January 2013 to August 2015) were retrieved from the medical records and analysed to comprise the control group (CG). The local standards for the CG included fluid maintenance of 5–10 mL kg-1 h-1 of lactated Ringer’s solution during surgery plus fluid replacement for fasting (2 mL kg-1 h-1) in the first 3 h and routine basic monitoring using five-lead electrocardiogram, pulse oximetry, invasive or noninvasive blood pressure monitoring, and capnography. Noradrenaline was used if the MAP were persistently < 65 mm Hg after volume expansion with 1 L of lactated Ringer’s or saline. Dobutamine was used if there were signs of deranged perfusion, which was indicated by altered ScvO2 or lactate, despite fluid replacement.
Patients in the IG were considered to have achieved the goal if the CO2 gap was < 6 mm Hg at T2. Postoperative complications were defined by the International Surgical Outcomes Study, and only moderate and severe complications were considered [15]. Patients were followed-up for complications during hospitalisation and for 180-day mortality.
Outcomes
The primary endpoint was the occurrence of moderate or severe postoperative complications. The secondary endpoints were ICU length of stay and mortality rates at 30, 90, and 180 days.
Statistical analysis
We assumed that moderate or severe complications would occur in 40% of the CG. At a significance level of α = 0.05, with regard to the primary outcome, 102 patients would be required in each group in order to have an 80% chance of detecting a 20% difference between the CG and IG.
Normally distributed data were presented as mean ±standard deviation (SD), whereas non-normally distributed data were presented as median (interquartile range) or as a percentage of the group from which they were derived. For categorical data, the χ2 test was used to compare qualitative data. Qualitative and quantitative data were compared using Student’s t-test or analysis of variance (ANOVA), when normally distributed, or the Kruskal-Wallis test in other circumstances. All statistics were two-tailed and P ≤ 0.05 was considered to be significant. Relative risk (RR) and 95% confidence interval (CI) were calculated to measure the differences in the number of complications between groups. Kaplan-Meier curves were generated to assess survival time. The statistical analysis software used was SPSS Statistics v25.0 (IBM®).
RESULTS
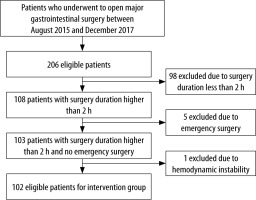
Of 394 eligible patients who underwent open major gastrointestinal surgery, 204 were included in the study. Of 206 eligible patients evaluated between August 2015 and December 2017, 102 patients were included in the IG after exclusion of 104 patients because of surgery duration of less than 2 h (n = 98), emergency surgery (n = 5), and haemodynamic instability (n = 1), as shown in Figure 2.
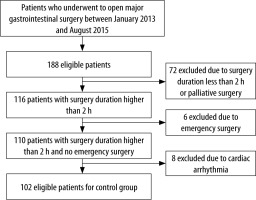
Data from 102 patients in the CG were retrieved from the electronic medical records of a cohort of 188 patients who underwent surgery between January 2013 and August 2015. Eighty-six patients were not included in the analysis or were excluded afterward because of surgery duration of less than 2 h or palliative surgery (n = 72), emergency surgery (n = 6), and cardiac arrhythmia (n = 8), as shown in Figure 3.
The patients’ characteristics are detailed in Table 1. Compared with the CG, the IG underwent more esophagectomy procedures and less gastrectomy procedures. There were no differences between the IG and CG in the P-POSSUM-predicted mortality and in the duration of surgery.
TABLE 1
Patients’ characteristics
The laboratory, haemodynamic, and perfusion variables are detailed in Table 2. The CO2 gap (in mm Hg) was significantly lower in the IG than in the CG at T1, T2, and T3 and increased in both groups at T4, particularly in the IG. The ScvO2 values were significantly higher in the IG than in the CG at T1, T2, and T3. Haemoglobin was higher and O2ER was lower in the IG than in the CG throughout all time points. Serum lactate (mEq L-1) was significantly higher in the CG than in the IG at T1. At T4, there were no significant differences between groups in the values of lactate, ScvO2, and oxygen extraction ratio (EO2R), but the base excess was lower in the IG than in the CG.
TABLE 2
Comparison between control group (CG) and intervention group (IG) laboratory, haemodynamic, and perfusion data
Both groups received not significantly different volumes of crystalloids, but compared with the CG, the IG received a significantly lower volume of colloids during surgery and had significantly lower urine output. There was no difference in the use of dobutamine between groups, but noradrenaline was administered more frequently in the IG than in the CG (Table 3). The proportion of goal achievers was higher in the IG than in the CG at T2 (46% vs. 31%, RR = 1.50, 95% CI: 1.005–2.238; P < 0.05) and at T3 (51% vs. 32%, RR = 1.59, 95% CI: 0.930–2.708; not significant).
TABLE 3
Comparison of the therapeutic interventions between the control group (CG) and intervention group (IG)
As shown in Table 4 and 5, the rates of moderate and severe postoperative complications were lower in the IG than in the CG. Except for more moderate AKI, defined as increase of creatinine 2.0–2.9 fold from baseline within 7 days or ≤ 0.5 ml-1 kg-1 h over 12 hours.
TABLE 4
Primary and secondary outcomes
TABLE 5
Comparison of the moderate and severe complications between the CG and IG
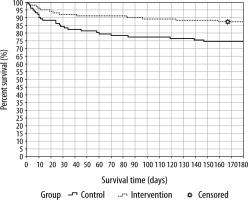
Fewer complications per patient occurred in the IG than in the CG. No differences between groups were observed with regard to days on mechanical ventilation and length of stay in the ICU or hospital. The respective mortality rates were lower in the IG than in CG, with statistical significance at 90 and 180 days. The 180-day Kaplan-Meier survival curve is shown in Figure 4.
DISCUSSION
The main findings of this study were 1) a 53% reduction in the risk of having a moderate or severe postoperative complication in the IG than in the CG, 2) lower mortality rates at 90 and 180 days in the IG than in the CG, 3) PGDT improved peripheral tissue perfusion during surgery, and 4) the use of noradrenaline was more frequent in the IG than in the CG.
In these high-risk oncological surgical patients, there was a significant decrease in the likelihood of having a moderate or severe complication after the implementation of an algorithm for intraoperative haemodynamic management. Postoperative complications have been known to have a huge impact on the short- and long-term survival, costs, and quality of life [9]. Data from 105,000 surgical patients showed that the occurrence of one complication after surgery was an independent predictor of mortality and reduced the likelihood of long-term survival by 69% [16]. Other authors have reported the negative impact of postoperative complications on long-term outcomes [17, 18]. In our study, the significant effect on mortality was probably associated with the impact on severe complications.
At all-time points during surgery, the CO2 gap was lower in the IG than in the CG, and there was a 50% higher likelihood of achieving a CO2 gap of < 6 mm Hg in the IG than in the CG. These results suggested that the protocol was feasible and had good adherence. In this study, the CO2 gap was used as a surrogate marker of cardiac output for intraoperative haemodynamic management and enabled the determination of improved perfusion, as shown by the higher ScvO2 and lower O2ER in the IG than in the CG.
SvO2 reflects the adequacy of oxygen delivery, in relation to oxygen consumption. Futier et al. proposed that a CO2 gap < 5 mm Hg serves as a complementary tool to ScvO2 during resuscitation, when the ScvO2 is higher than 70%, such as during cytopathic hypoxia or microcirculatory alterations, which result in decreased oxygen extraction [8]. Indeed, during deep sedation or anaesthesia, metabolism is decreased and oxygen extraction is altered. Therefore, the CO2 gap might serve as a complementary target to identify inadequacy of fluid therapy after a major abdominal surgery. Other authors have demonstrated the associations of low SvO2 and high CO2 gap upon ICU admission with more organ dysfunction and complications after major gastrointestinal surgeries in high-risk patients, and the application of interventions that addressed these variables led to better outcomes [8, 19].
Interestingly, noradrenaline was more frequently used in the IG than in the CG, although no difference in MAP was seen between the two groups. The increased use of noradrenaline was probably due to the intervention algorithm, in which fluid therapy was guided by PPV. A recent study demonstrated that the use of noradrenaline to maintain systolic blood pressure within 10% of the patient’s baseline was related to improved results and less organ dysfunction. This result was probably secondary to increased venous return due to enhanced blood redistribution from the unstressed to the stressed blood volume [20]. Early use of vasopressors during anaesthesia induction is likely to decrease the occurrence of hypoperfusion in the gut, brain, and kidneys and prevent a cycle of inadequate blood flow redistribution and tissue hypoperfusion.
Although previous studies on cardiac output measurements have used dobutamine more often during PGDT, in this study, noradrenaline was more commonly used than dobutamine (47% vs. 18%) among patients in the IG.
The treatment algorithm for the IG recommended maintaining a serum haemoglobin level > 10 mg dL-1.
Red blood cells were transfused in 19.6% of the patients in the IG and in 43.1% of those in the CG. The relatively high haemoglobin level despite the relatively low transfusion rate in the IG can be explained by the precise PPV-guided fluid therapy and the associated frequent use of noradrenaline in this group. The use of noradrenaline decreases fluid responsiveness by redistributing blood from the unstressed to the stressed blood volume. In addition to decreased fluid responsiveness, haemodilution was less and haemoglobin levels increased with PPV-guided fluid infusion.
One point of concern is the incidence of moderate acute kidney injury in the IG that was greater, maybe due to a more restrictive fluid therapy in this group. The amount of fluids in the IG was less than in the CG, but without statistical significance.
Some limitations of our study must be addressed. Before-and-after studies are inherently susceptible to bias and cannot exclude the possibility of a Hawthorne effect. To reduce the effects of trends or investigator bias, we minimised the time span between the two cohorts by including consecutive surgical patients in the study group before the introduction of the new treatment protocol. There was some imbalance between groups, with more patients undergoing esophagectomy procedures in the PGDT group; however, this must be not a problem because more complications and higher mortality are expected in patients submitted to this procedure. Nevertheless, without randomisation, the results must be interpreted with caution. Finally, the study was performed in a particular population of major gastrointestinal surgeries. Therefore, these results may not be generalisable to other types of surgeries.
To our knowledge, the present study was the first to use the CO2 gap as a therapeutic target in addition to PPV during PGDT in high-risk patients undergoing gastrointestinal surgeries. The introduction of haemodynamic therapy with PPV and CO2 gap was associated with decreased rate of severe complications and significantly lower postoperative mortality.
In conclusion, the use of a haemodynamic protocol that aimed to normalise the CO2 gap and PPV seemed to be feasible and effective in reducing severe complications and mortality in patients undergoing major gastrointestinal surgeries. It may be a simple alternative to more complex monitoring, particularly in low-resource settings, and may provide timely and reliable information to guide therapy.