Dear Editor,
This letter discusses the efficacy of current antiviral therapy used in severe COVID-19 infection. Since the first severe cases were documented, several antiviral options have been studied as adjuncts to standard supportive care [1, 2]. Firstly, the combination of lopinavir–ritonavir resurrected from SARS and MERS outbreaks and soon abandoned after the publication of several trials like the randomized controlled trial RECOVERY, which concluded that it was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progression to invasive mechanical ventilation or death [3]. Remdesivir is currently the only antiviral agent approved for the treatment of COVID-19. It is recommended for use in hospitalized patients who require supplemental oxygen. However, it is not routinely recommended for patients who require mechanical ventilation due to the lack of data showing any benefit at this advanced stage of the disease [4–6].
We report a multicentre observational study whose aim was to access the efficacy of these antiviral options in severe COVID-19 pneumonia using an analysis of the viral load throughout treatment with real-time polymerase chain reaction (PCR). We sequentially included all patients with confirmed COVID-19 infection admitted to the intensive care medicine departments of 2 hospitals in central Lisbon (Portugal) from March to September 2020. Patients were divided into 3 groups according to the clinical protocol in force at their time of admission, as seen in Figure 1. Exclusion criteria were pregnancy, known allergy or hypersensitivity to lopinavir–ritonavir or remdesivir, QT prolongation, alanine aminotransferase or aspartate aminotransferase levels > 5× the upper limit of the normal range or acute kidney injury higher than grade 2 according to KDIGO criteria. Electrocardiogram monitoring (in particular, QT interval), was performed continuously in all patients. SARS-CoV-2 RNA detection was performed by real time-PCR from pharyngeal swabs/endotracheal aspirates using primers for genes ORF1a, N, and E. The number of thermal cycles needed for RNA detection (threshold cycle – Ct) was recorded. RNA viral load was indirectly estimated by Ct count and considered undetectable above 40 thermal cycles. Local Ethical Committee and Board Administration, Comissão de Ética para a Saúde e Conselho de Administração do Centro Hospitalar Universitário de Lisboa Central, approved this study and its publication (INV_69_854/2020); written informed consent was waived by the ethics committee.
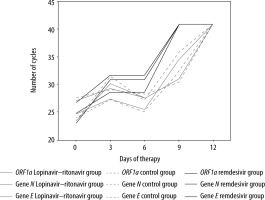
A total of 128 patients were included in this cohort and divided in 3 groups according to their treatment regimen. Among all cohorts, the majority were male with a high prevalence of more than 2 comorbidities (Table 1), which is in line with the literature [7]. No relevant differences were found between baseline characteristics among all groups. Time to hospital and intensive care unit (ICU) admission were also similar in all groups. Differences in the need for mechanical ventilation may be due to our early approach and early orotracheal intubation protocol implemented in the first part of this pandemic. Furthermore, as the understanding and knowledge of COVID-19 disease has become more extensive, new options such as high-flow oxygen therapy allow us to reduce the number of invasive mechanical ventilation patients. Time to negative SARS-CoV-2 was slightly lower in the remdesivir group (16 vs. 14.6 days for the lopinavir–ritonavir and remdesivir group, respectively). As described by Wang et al., we used cycle threshold values of real-time PCR as indicators of the number of viral copies [8, 9]. When possible, the cycle count for gene detection using real-time PCR was performed using endotracheal aspirates/bronchoalveolar lavage. As seen in Figure 2, before any treatment, the median Ct count was similar in all groups. During treatment the remdesivir group seemed to reach a negative search earlier than lopinavir–ritonavir and control groups, as can be seen with a progressively higher cycle count number (Figure 2). This conclusion was identical to those described by Cao et al. [10], and was in line with the multicentre trial developed by Wang et al. [11] in which remdesivir did not provide significant clinical or antiviral effects in seriously ill patients with COVID-19. ICU mortality was higher in the remdesivir group (31.3%).
TABLE 1
Demographic and clinical characteristics
Managing SARS-CoV-2-infected patients has truly been a medical challenge. Despite the global vaccination program in course, we suggest that it is important not to lose the focus on antiviral therapy because it can have the greatest impact before the illness progresses into the hyperinflammatory state that can characterize the later stages of disease, including critical illness.