Introduction
Allergen immunotherapy (AIT) is the only disease-modifying therapy for allergic rhinitis, allergic asthma, and hypersensitivity to Hymenoptera venoms confirmed in clinical trials, case reports, and meta-analyses [1–4]. AIT-induced tolerance includes a change from Th2 to Th1 response, increased regulatory T and B cells, proinflammatory effector cell downregulation and IgE suppression. AIT also stimulates the production of IgG4, IgA and IgD. AIT may also induce a decrease in group 2 of innate lymphoid cells (ILCs) and increased ILC1 and ILC3s [2, 5]. The safety and efficacy depending on the desensitised allergens are presented in international consensuses and, most importantly, in the EAACI recommendations [6, 7].
Even with the proven evidence for the short-term benefits of AIT, the interest of physicians and patients is focused on the long-term benefits of such treatment [6, 7]. An often-overlooked group in the context of this problem is elderly patients. Evidence shows that using AIT in seniors is effective and safe in patients with allergic rhinitis who have insufficient symptomatic pharmacotherapy [7–10]. However, there are only a few observations of long-term benefits in this group.
Aim
The study aimed to prospectively assess the long-term benefits of injection and sublingual AIT in patients over 60 with rhinitis and allergy to house dust mites (HDM) from the perspective of 7 years.
The primary endpoint was the change from baseline in the mean average adjusted symptom score (AAdSS) difference in the label compared to placebo during 7 years of follow-up after AIT.
The second endpoints were changes in quality of life based on the questionnaire RQLQ and the immunological response in IgG4 to D. pteronyssinus and D. farinae after AIT during follow-up.
Material and methods
Study design
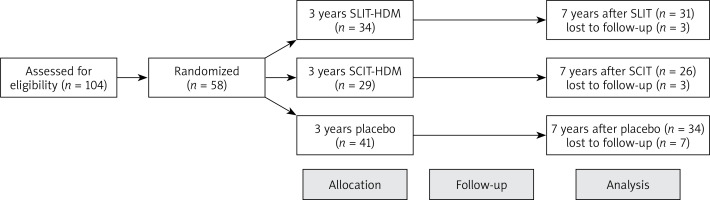
It was a randomised, double-blind placebo-controlled and observational study. Patients after discontinuation of 3 years of AIT were observed during the next 7 years to assess the sustained clinical effect of treatment.
Patients
Forty-seven patients with perennial allergic rhinitis (65.8 ±4.9 years old) after 3 years of sublingual allergen immunotherapy (SLIT) for HDM with the use of Staloral mites or placebo and 29 similar patients (62.3 ±3.1 years old) after 3 years of perennial injection allergen immunotherapy (SCIT) for HDM with the use of Purethal mites or placebo were observed during next 7 years (Figure 1). Registered commercial indications used desensitisation vaccines. Symptoms and medication scores were calculated as AAdSS. Quality of life with rhinitis was monitored with the use of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). Serum allergen-specific IgG4 to D. pteronyssinus and D. farinae were measured every year of follow-up. All these data were compared to the placebo group. In this study, all participants were monosensitised to the extract of D. pteronyssinus and D. farinae. The characteristics of all participants are shown in Table 1.
Table 1
Characteristics of patients prior to allergen immunotherapy
| Parameter | SLIT (n = 31) | SCIT (n = 29) | Placebo (n = 34) |
|---|---|---|---|
| Age [years] | 63.5 ±1.9 | 61.5 ±2.9 | 65.1 ±4.1 |
| Female (%) | 67 | 71 | 62 |
| Duration of rhinitis before AIT [years] | 4.5 ±2.5 | 3.8 ±3.7 | 4.1 ±2.4 |
| Number of subjects with asthma | 3 | 3 | 2 |
| Number of smokers | 6 | 5 | 9 |
| Mean weekly nasal symptom score | 3.78 ±1.28 | 3.96 ±1.91 | 4.1 ±1.7 |
| Mean weekly medication score | 1.45 ±0.45 | 1.8 ±0.42 | 1.2 ±0.77 |
| Total IgE | 201.83 ±78.3 | 189.3 ±67.41 | 190.1 ±93.5 |
| Specific IgE to Der p [kU/l] | 18.9 ±5.31 | 21.03 ±8.21 | 19.4 ±3.6 |
| Specific IgE to Der f [kU/l] | 14.18 ±4.32 | 11.4 ±6.44 | 20.8 ±9.33* |
Intervention
The randomisation procedure with random selection relied on the computer program. The first group of patients received Purethal or a placebo (extract of mites 20,000 AUeq/ml, HAL Allergy, Leiden, The Netherlands). The product contains Der p1 = 14.0 μg/ml and Der p 2 = 20.0 μg/ml. Purethal was administered using the following regimen: first dose – 0.1 ml, second dose – 0.2 ml, third dose – 0.5 ml every week, and then 0.5 ml every 4–5 weeks for 36 months. The average cumulative quantity of 740,500 BAU was reached. The second study group was treated for 3 years using Staloral 300 SR (extract of D. pteronyssinus, D. farinae: 50/50%; Stallergenes Greer, London, UK) or placebo. First, the patients received increasing doses of 1–8 puffs of the 100 IR/ml extract daily. Then the patients took 1–8 puffs of the 300 IR/ml extract, and after that, they received maintenance treatments consisting of 18 puffs (1 puff = 30 IR) of 300 IR/ml extract five times a week for 36 months. Using this schedule, an average cumulative dose of 645 200 IR of allergens was administered.
Symptomatic treatment
The following drugs were permitted: antihistamine (5 mg levocetirizine), intranasal corticosteroid drops (mometasone), ocular antihistamine drops (ebastine) and oral steroid (5 mg tablet of encortolon).
Scoring
Patients were monitored for allergic clinical symptoms and medication use from July 2012 to June 2022.
All participants monitored nasal symptoms regarding weekly medication during the observation, including 3 years of the AIT and 7 years of follow-up. Each day, the patient assessed daily the severity of each symptom (sneezing, rhinorrhoea, nasal congestion and pruritus) on a four-point scale: 0 points for lack of symptoms, 1 point for mild symptoms, 2 points for moderate symptoms, 3 points for severe symptoms.
The analysis of symptomatic therapy was as follows: one point for antihistamines, two points for nasal corticosteroids and three points for oral corticosteroids [11]. Patients completed a diary every week between the screening visit and their last visits during the trial. Weekly symptoms and medication scores were monitored, after which mean monthly scores were calculated.
Symptoms and medication scores were presented as AAdSS. It is the average total score of four rhinitis symptoms adjusted for rescue medication use. The AAdSS was confirmed as a primary endpoint in AIT trials [12].
Quality of life
The patient’s quality of life was scored using the RQLQ for adults every year during the observation [13].
Allergen-specific IgG4
After AIT and every year during observation, serum-specific IgG4 levels to the extract of D. pteronyssinus and D. farinae were determined by Immuno Uni CAP (ThermoFisher Scientific, Uppsala, Sweden).
Statistical analysis
Statistical analysis was performed using Statistica version 8.12 (SoftPol, Cracow, Poland). The ANCOVA test calculated the mean AAdSS score difference in the label compared to the placebo. The c2 test was used to analyse changes in RQLQ scores. IgG4 was calculated as the baseline’s least squares mean (LS mean) change. Differences were considered significant at p < 0.05.
Results
Clinical efficacy
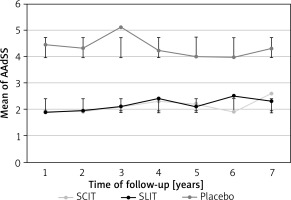
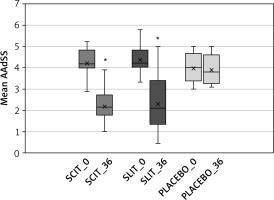
After 3 years of AIT for HDM allergy, a significant clinical effect was observed in both groups based on AAdSS compared to the baseline and the placebo group (Figure 2). After 7 years of follow-up, there was a sustained trend of decreased clinical symptoms of allergic rhinitis in desensitised patients relative to placebo. No significant differences were observed between SCIT and SLIT (Figure 3).
Figure 2
Changes in AAdSS after 3 years of allergen immunotherapy to house dust mites
SCIT_0 – start of injection allergen immunotherapy to house dust mites (HDM), SCIT_36 – after 36 months of therapy, SLIT_0 – start of sublingual allergen immunotherapy to house dust mites (HDM) SLIT_36 – after 36 months of therapy, PLACEBO_0 – start, PLACEBO_36 -–after 36 months, *significant decrease of AAdSS after allergen immunotherapy in both groups in comparison to the start and to compare with placebo (p < 0.05).
Quality of life
After 7 years of follow-up, quality of life based on RQLQ score was significantly decreased in patients who received AIT from 1.82 (95% CI: 1.54–1.92) at the start to 1.28 (95% CI: 1.13–1.47) for SCIT and 1.94 (95% CI: 1.63–2.1) to 1.41 (95% CI: 1.21–1.63) for SLIT (p < 0.05).
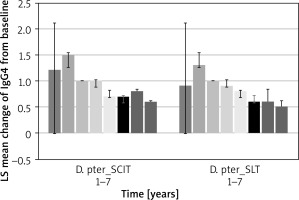
Serum allergen-specific IgG4
Serum IgG4 values after the end of desensitisation gradually decreased. However, they remained at a significant level (Figure 4).
Discussion
The long-term effects of allergen immunotherapy for HDM have been the subject of several recent studies [14–17]. Fritzsching et al., in a real-life study of patients with allergic rhinitis and asthma, showed the long-term and lasting effectiveness of AIT-HDM during 9 years of observation [15]. It was associated with a reduced likelihood of using symptomatic medications, asthma exacerbations, and a change in respiratory hospitalisations. Our much smaller study found similar results in reducing the use of symptomatic drugs and symptoms in allergic rhinitis. However, in the presented study, most patients did not have asthma at baseline, and in the cited study, patients were younger.
The question is whether, despite immune ageing, such long effectiveness after AIT is possible in older patients. In the context of the efficacy of AIT, ageing of the immune system may reduce the ability of the immune system to respond to new antigens, promote the accumulation of memory T cells, significantly lower the number of CD4(+) and CD8(+)CD28(+) cells, induce increased numbers of CD8(+)CD28(–) apoptosis-resistant cells and cause an inversion in the ratio of CD4/8 T cells [18, 19]. Overall survival is worse in patients with inversion of the CD4/CD 8 T-cell ratio, reduced proliferative response to mitogenic stimuli, and severely reduced B cell numbers. However, these quantitative changes do not appear to strongly impact the induction of allergen tolerance with AIT [18, 19].
The first study, which focused on long-term benefits after AIT based on observation of thirty-eight elderly patients (mean ± standard deviation, 66.2 ±2.7 years old) who received preseasonal SCIT or placebo for grass pollen allergy were monitored for 3 years and compared with a placebo group. The combined symptom medication score decreased and sustained less during the observation [20]. Although the study concerned a different allergen and a different administration protocol, the results of long-term clinical improvement are consistent with ours. So far, the presented observation has been the longest, showing the long-term effectiveness of older patients after AIT.
As the results show, also in older people with rhinitis only, a reduction in new allergic asthma incidents after AIT can be expected, as in other observations [16]. However, this phenomenon has a smaller scale due to the age of patients and the lower risk of asthma de novo in them. This is evidenced by sustained clinical improvement with a reduction in treatment use and maintaining a good quality of life, and the presence of the IgG4 biomarker. These observations are consistent with a similar study on SLIT-HDM, but the observation time is shorter there [21]. In addition, the comparative effectiveness of two AIT methods in seniors for the same allergen in similar clinical groups was shown for the first time. It confirms previous evidence of equivalent efficacy in younger age groups.
The study was limited by relatively small groups of observations, which results from the limited group of patients predisposed to this type of treatment due to age and various contraindications to AIT. Only a limited number of parameters were analysed in the study, resulting from the desire to limit too much burden on patients during such an extended observation period.