Purpose
Cervical cancer is a common cancer in women in India and more than 2/3 of patients present at an advanced stage. Recent data of the National Cancer Registry Programme (NCRP) shows that the incidence accounts for 15.7% of all cancers [1,2]. Radical radio-chemotherapy, including external beam followed by brachytherapy (BT), is a standard of care. Brachytherapy forms the cornerstone for local control rates and toxicities [3,4]. Brachytherapy is generally applied as high-dose-rate (HDR) fractionated treatment delivered by intracavitary (IC) alone, or IC and interstitial or interstitial alone. Brachytherapy offers the advantage of delivering a very high-dose to the tumor and low doses to adjacent organs at risk (OARs).
Recently, a paradigm shift in BT practice from conventional planning to image-based brachy approach was implemented. Image-based target volumes related to disease at the time of BT have been published by GEC-ESTRO [5,6]. Tumor volume is well recognized as one of the most important prognostic factors in terms of local control. Magnetic resonance (MR) imaging-based definitions are the gold standard and have shown promising results in many mono-institutional series and a multicenter study [7]. MR-based BT approach mandates the use of MR-compatible applicators, MR imaging, and adaptive planning to achieve optimal doses to target and OARs sparing. There is an ongoing research in terms of variety of applicators and optimization techniques to achieve better local control and sparing of OARs. Han et al. has shown better sparing of OARs with the use of novel direction modulated brachytherapy (DMBT) tandem applicator in small volume disease [8].
This feasibility study evaluates the use of plastic polyether ether ketone (PEEK) catheters to initiate MR-guided BT and report the preliminary experience of utilizing plastic catheters for MR image-guided BT approach for advanced cervical cancer.
Material and methods
This study includes locally advanced and post-operative cervical cancer patients. As a pilot study, five patients were included and among them, three were FIGO stage IIIB, one post-operative, and one post-operative recurrent case. All these patients underwent standard pre-treatment evaluation of complete history, physical examination, and examination under anesthesia (EUA). Cystoscopy, proctoscopy, routine hematological, and renal function tests were performed. Imaging included chest radiography, computed tomography (CT) scans of abdomen and pelvis, and bone scan if clinically indicated. Patients received external beam radiotherapy dose of 50 Gy in 25 fractions to whole pelvis using 3D conformal therapy. If the gross tumor volume (GTV) at the time of brachytherapy was more than 4 cm with the parametrial extension, interstitial brachytherapy was performed using PEEK catheters with Syed-Neblett template. CT scan images were acquired with 3 mm axial slices and treatment planning was performed using BrachyVision TPS (Varian Medical Systems, Palo Alto, CA, USA) with volume optimization.
Recently, in our center, 1.5 Tesla MRI device was implemented into the clinical use. However, MR-compatible brachytherapy applicators were not available; therefore, the implant procedure was performed with semi-flexible thermoplastic PEEK catheters and Syed-Neblett template. This plastic catheter usage is prevalent in prostate and breast brachytherapy; the catheters are biocompatible and the whole applicator set is MR-compatible. The thermoplastic PEEK catheters used in our study were manufactured in India.
T1 and T2 relaxation MR images were acquired for volume delineation and planning; CT images were also obtained to visualize the catheters’ position by placing copper dummies in the catheters. The DICOM (digital imaging and communication in medicine) images of MRI and CT were imported into BrachyVision TPS for planning.
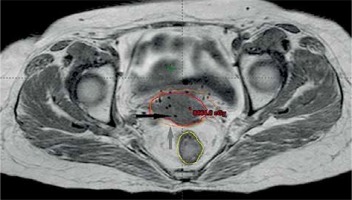
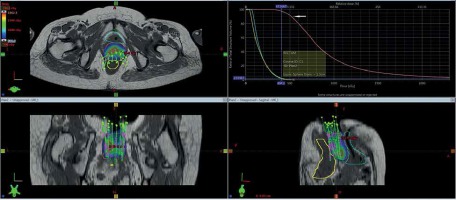
Gross tumor volume, high-risk clinical target volume (HR-CTV), intermediate-risk clinical target volume (IR-CTV), and OARs were delineated with the use of MR T2 weighted images, following GEC-ESTRO guidelines. HR-CTV delineation was challenging, as pre-brachy MR images were not available. This was overcome by changing the contrast resolution and EUA findings (Figure 1). T1 weighted images were helpful for a catheter reconstruction, and the catheters were better appreciated as black straight lines without any interruptions. Haack et al. showed that plastic catheters are well suited for MR-based reconstruction in T1 images and all catheters were visible without artifacts [9]. Depending of the extent of disease, the active lengths of the implant ranged from 6-8 cm. The dose prescribed was 20 Gy in 4 fractions (30 Gy low-dose-rate [LDR] equivalent [EQD2]) to HR-CTV, 6 hours apart, and delivered over two days. Doses of 2 cc of bladder and rectum were restricted to less than 4.0 and 3.7 Gy per fraction, respectively. With our initial experience, the dose escalation was considered to be 6 Gy per fraction. Treatment plans were performed using volumetric optimization with 5 mm dwell position (Figure 2). The OARs constraints were met using manual dragging of isodose lines. The treatment was executed using 192Ir Gammamed Plus HDR remote afterloading machine (Varian Medical Systems, Palo Alto, CA, USA), with 192Ir high activity of 10 Ci max, source of 4.6 mm active length and 0.9 mm in diameter.
Results
The prescribed dose to HR-CTV was 5 Gy × 4 fractions (30 Gy LDR equivalent). The dose to HR-CTV and OARs were analyzed using DVH. Dose values were biologically normalized to equivalent doses of 2 Gy fractions (EQD2, equivalent to 50 cGy/h LDR, using spreadsheet of the Medical University in Vienna) by applying the linear-quadratic model. The 3D dose-volume parameters for five patients are shown in Table 1. The average tumor volume was 67 cc, the average dose to 90% of HR-CTV (D90) volume was 5.7 Gy (79 Gy EQD2), and the average dose to 100% of HR-CTV (D100) volume was 3.8 Gy (68 Gy EQD2). The average doses of 2 cc of rectum and bladder were 3.71 Gy and 4.04 Gy, dose maximum were 70 Gy and 76 Gy EQD2, respectively. High-dose volumes V150 and V200 were 28 cc and 14 cc, respectively, with close proximity to the catheters.
Table 1
Dose-volume parameters for HR-CTV and organs at risk (OARs)
| HDR (Gy/#) | EQD2 (2 Gy equivalent) | Tumor volume in cc | D90 cGY (EQD2) | D100 cGY (EQD2) | V150 [cc] | V200 [cc] | Rectum 2 cc dose | Total 2 cc rectal dose (HDR dose) | Bladder 2 cc dose | Total 2 cc bladder dose (HDR dose) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 × 4 | 25 | 55 | 594 | 378 | 37 | 22 | 346 | (17) 67 | 413 | (23) 73 |
| 5 × 4 | 25 | 62 | 496 | 359 | 29 | 12 | 314 | (16) 66 | 342 | (17) 67 |
| 5 × 4 | 25 | 89 | 488 | 325 | 17 | 8 | 359 | (19) 69 | 364 | (19) 69 |
| 6 × 3, 5 × 1 | 28 | 62 | 690 | 522 | 30 | 14 | 483 | (28) 78 | 527 | (33) 83 |
| 6 × 3, 5 × 1 | 30 | 65 | 516 | 317 | 25 | 12 | 355 | (18) 68 | 375 | (20) 70 |
| AVG | 26.5 | 67 | 557/29 | 380/18 | 28 | 14 | 371 | (20) 70 | 404 | (26) 76 |
Discussion
Image-guided brachytherapy is the standard of care, either in the form of orthogonal X-rays for 2D or CT imaging for the 3D volume-based brachytherapy. The volumetric imaging is superior than the planar imaging in terms of doses to tumor and OARs [10,11]. Tumor volume coverage with an adequate dose is important prognostic factor for better local control rates. Standard intracavitary brachytherapy does not cover the tumor volume adequately in locally advanced disease and in distorted anatomy [12]. Syed et al. developed a technique of interstitial/intracavitary brachytherapy to improve the dose distribution in patients with locally advanced disease [13], and along with Aristizabal et al. have reported good results using similar techniques [14,15].
Routinely at our center, interstitial brachytherapy (ISBT) is applied for locally advanced cancer cervix patients using Syed-Neblett template with stainless steel needles, and treatment volume is planned based on EUA findings. Initially, the treatment plans were performed on X-ray planar images and later on moved to CT images.
Dimopoulos et al. published the utility of MR imaging in tumor delineation in cancer cervix. The GEC-ESTRO Working Group (IV) guidelines stated the basic principles and parameters for MR multi-planar image in image-based adaptive brachytherapy for cancer cervix [16]. Vienna group of Pötter et al. [7] has developed ring applicator for intracavitary brachytherapy. Learning form failures in controlling the parametrial disease, the Vienna group modified the ring applicator to Vienna II applicator, with interstitial facility to adequately cover the HR-CTV. They demonstrated that the image-guided adaptive brachytherapy (IGABT) using Vienna II intracavitary/interstitial (IC/IS) applicator has led to a significant improvement of local control in locally advanced cervical cancer (LACC) patients, when comparing to previous results. Derks et al. in a retrospective study has shown the effect of 2D conventional brachytherapy (CBT) compared to 3D MRI-guided brachytherapy (IGBT) with and without the use of interstitial needles, showing an improved local control and reduced toxicities [17].
The GYN GEC-ESTRO Working Group guidelines (I and II) focus on 3D dose-volume parameters for brachytherapy of cervical carcinoma. They recommend D90, D100, and dose of 2 cc to OARs [5,6].
Many oncologic centers lack the facility for MR image-guided brachytherapy. Since our center has the 1.5 Tesla MR, we are in the process of acquiring MR-compatible intracavitary/interstitial (IC/IS) brachytherapy applicators. To overcome the lack of facility, a new technique was implemented using Syed-Neblett template with PEEK catheters for interstitial implantation. The whole ISBT set was made MR-compatible. These catheters are firm with a sharp tip and are inserted using a stainless-steel stylet that facilitates the catheter to pass through the tumor and minimizes the convergence of catheters at cranial end.
In the first patient, the CT planning was performed using copper dummies to delineate the catheters. The dose-volume optimization was done in CT images, and later on MR image sequences that were acquired for planning. The identification of catheters and catheter tracking was not difficult, but the delineation of GTV was problematic due to implant geometry and contrast resolution as well as pre-treatment MR images not available. The HR-CTV delineation was completed using contrast resolution in T2 sequences and findings from clinical examination under anesthesia and to some extent, with initial CT findings. The patient comfort with plastic catheter was excellent in terms of pain.
In the initial three cases, the prescription dose was 20 Gy in 4 fractions to the HR-CTV, which was 30 Gy LDR equivalent, as this being the institute’s practice. The average HR-CTV (D90) was 67 cm3 and was adequately covered with an average dose of 5.57 Gy; dose to 100% HR-CTV (D100) was 3.8 Gy per fraction. As per GEC-ESTRO recommendations, 2 cc of bladder and rectal doses were 3.71 Gy and 4.04 Gy, respectively, which were 70% and 85% of the prescribed dose, respectively. The total doses of 2 cc of rectum and bladder were 70 and 76 Gy, respectively (Table 1). With our initial experience, the dose escalation was considered to be 6 Gy/fraction for 3 fractions. High-dose volumes, V150 and V200, were 28 cc and 14 cc, respectively, with close proximity to the catheters. As this is our initial experience with PEEK catheters used for interstitial implant, the dose to HR-CTV was restricted to 80 Gy EQD2. With long-term follow-up and depending on the toxicity profiles, further dose escalation to HR-CTV could be considered.