Purpose
Colorectal cancer is the second leading cause of cancer-related deaths [1]. In patients with metastasized colorectal cancer and a curative intended therapy setting, primary resection of liver metastases is the gold standard. However, in patients with oligo-metastases and contraindications for abdominal surgery, other treatment options may be considered in a multidisciplinary setting [2, 3], such as thermal ablation: microwave ablation (MWA) and radio-frequency ablation (RFA), high-conformal ablative radiotherapy: stereotactic body radiation therapy (SBRT) and high-dose-rate brachytherapy (HDR-BT), and loco-regional therapies, including trans-arterial chemoembolization (TACE) and selective internal radiation therapy (SIRT). Different from thermal ablation techniques, HDR-BT has no limitations regarding tumor size or proximity to vascular structures [4, 5]. This technique uses a single-fraction irradiation, with an iridium-192-source directly placed in target volume via percutaneously inserted catheters [2, 6].
In a study among 73 patients with colorectal metastases previously treated with liver resection and chemotherapy, HDR-BT treatment showed a median survival of up to 23.4 months [2, 7]. In order to further establish this local ablation technique, studies on safety, possible complications, and long-term effects are required.
For liver biopsy and thermal ablation, needle track seeding has been reported in case reports and a few studies [8-14]. Majority of research on this topic pays attention to needle track seeding in patients with hepatocellular carcinoma (HCC) [15-20], and in general, its’ occurrence in patients with HCC is rare.
As to MWA, in HDR-BT, the procedure of percutaneously inserting catheters also carries a risk of needle track seeding. A study on needle track seeding in patients with HCC after HDR-BT detected a risk of 1.5% for needle track seeding in a catheter-based analysis of 588 catheter placements and an incidence of 9.0% in a patient-based analysis [21]. Denecke et al. [22] reported no needle track seeding after HDR-BT for treatment of HCC lesions in a transplant setting. Studies on the occurrence of seeding after biopsy in patients with colorectal metastases identified incidences of 10% to 16% in patient-based analyses [12-14]. Yet, larger studies on needle track seeding in patients with colorectal metastases and especially brachytherapy are missing.
In this retrospective study, we included patients who underwent at least one HDR-BT for treatment of colorectal liver metastases. We aimed to identify the general risk and possible risk factors for development of track seeding. Furthermore, we evaluated not only extra-hepatic, but also intra-hepatic needle track seeding.
Intra-hepatic seeding was classified as seeding alongside the catheter path within the liver parenchyma. Extra-hepatic seeding was defined as seeding alongside the catheter path in parenchyma adjacent to the liver, e.g., fatty tissue, muscle, skin. Intra-hepatic track seeding has rarely been investigated due to challenges of evaluating possible seeding within the liver parenchyma, as it could be confounded with independent development of new metastases. As a new approach, we used fusion software to diagnose intra-hepatic seeding more confidently.
Material and methods
In this retrospective study investigating the occurrence of needle track seeding after brachytherapy, we included patients who underwent interstitial HDR brachytherapy for colorectal metastasis of the liver in an academic hospital from March 2006 to July 2010. Inclusion criteria were diagnosis of colorectal cancer with synchronous or metachronous metastasis of the liver deemed irresectable, legal age of 18 years, informed written consent, and at least one follow-up with CT or MRI after at least three months.
HDR brachytherapy technique
Interstitial brachytherapy is a local ablative technique, in which irradiation catheters are directly placed into target lesion under image guidance. Similar to RFA that uses high frequency current for application of heat after insertion of a probe in a target lesion, brachytherapy also carries the risk of spreading tumor cells during the insertion. First, the lesion was punctured with an 18 Ga coaxial needle, with a help of computed tomography (CT) or open magnetic resonance imaging (MRI) fluoroscopy, after which, a 6F diameter angiographic catheter sheath of 28.5 cm was placed using Seldinger’s technique. This was followed by insertion of an irradiation catheter of 35 cm with 6 CH into the soft catheter sheath, with the tip of catheter matching the end of sheath. The catheter was fixated by a single-suture. After insertion, diagnostic imaging was performed (e.g., contrast-enhanced CT) for development of a three-dimensional treatment plan. Afterwards, an iridium-192 source was placed into the lesion via the inserted irradiation catheters. Since the first position for placing the iridium source was 6 mm short of the catheter tip, slight over-penetration of the lesion rim was needed.
To achieve a complete ablation in the treatment of colorectal metastases, a surrounding dose of 25 Gy (clinical target volume – CTV) was preferable. For CTV calculation, gross tumor volume (GTV) was defined, and a 5 mm safety margin was added. Since the catheters were skin-fixated, CTV equaled to planning target volume (PTV), and no further safety margin was needed [6]. After single-fraction irradiation, the catheters and sheaths were removed and replaced by absorbable gelatin sponge in the track to prevent post-interventional bleeding. During this procedure, patients received individual, weight-adapted doses of fentanyl and midazolam for conscious sedation [6].
Follow-up
During routine follow-up after brachytherapy, consisting of CT or MRI-imaging at 6 weeks, 3 months, and 6 months after intervention, with following exams every 3 months, the patients were required to attend at least one follow-up visit not less than three months after therapy. CT protocol included an arterial, portal venous, and late venous phase, while MRI additionally included hepatobiliary imaging using a hepatocyte-specific contrast agent.
Imaging analysis
Since extra-hepatic track seeding after biopsy and local ablative therapies, such as RFA [8-14] in patients with colorectal metastases is known but not extensively studied, we aimed to analyze track seeding in patients who underwent brachytherapy. Different from other published studies, intra-hepatic seeding was especially analyzed.
After brachytherapy, all newly developed intra-hepatic and extra-hepatic lesions, which were located in the needle track, in close proximity of not exceeding 10 mm to the track, or not more than 10 mm distally from the former needle tip, were examined for needle track seeding. We chose a margin of 1 cm due to possible tissue shrinkage. Information on the size of lesions, number and location, total length of each catheter from skin level to the catheter tip were gathered as well as data on overshooting of target lesions beyond the distal rim. Two diagnostic radiologists evaluated all imaging data by consensus.
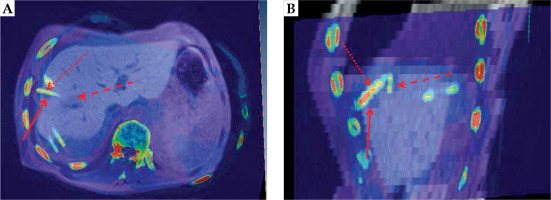
To objectify possible needle track seeding, Amira® 3.x software for the fusion of CT/MRI-imaging data from irradiation treatment and follow-up was used. Overlay images were created to identify the location of the suspected needle track seeding, with respect to the catheter position. An example for the image fusion is shown in Figure 1.
Statistical analysis
Imaging data and technical information on the catheter placement and irradiation procedure were recorded. Possible risk factors for development of needle track seeding, including patient’s demographics, tumor grading, synchronous or metachronous liver metastasis, and possible aspects of the catheter placement, such as number, kind of image guidance, and over-penetration of the catheter were identified.
For statistical analysis, SPSS® (IBM, Armonk, NY, USA) and SAS® (SAS Institute Inc., Cary, NC, USA) were applied. For metric variables, t-test or Wilcoxon-Mann-Whitney U-test were used; for evaluation of frequencies, χ2 test was applied. The influence of possible risk factors for the development of needle track seeding was evaluated using a generalized linear mixed model (GLMM). A p-value of ≤ 0.05 (two-sided) was considered statistically significant.
Results
Patients’ characteristics
A total of 138 patients were included in the study, with 85 males and 53 females. The median age at the time of first intervention was 68.8 years (range, 36-89 years) (Table 1). The patients were referred to our institution for salvage treatment of liver metastases. On average, the patients had 3.6 target lesions with a median of 3 (range, 1-7 lesions). All patients received at least one treatment with interstitial brachytherapy. In all cases, thermal ablation was not feasible due to tumor size (> 3 cm) or close proximity to the liver hilum or gastrointestinal organs. 472 liver lesions were treated, with 1,107 catheter placements.
Table 1
Patients and treatments characteristics, and analysis on influencing factors for needle track seeding (GLMM)
The majority (52.9%) of patients was diagnosed with colorectal cancer localized in the colon, followed by 38.4% of primary cancer at the recto-sigmoid junction, and only 8.7% in the rectal region. In the initial staging exam, the majority presented with lymphatic metastasis (29% N1, 36.2% N2) as well as distant metastases M1 (54.3%).
Most patients (95.7%) were treated with surgery for the primary tumor, which predominantly showed a G2 grading (moderately differentiated) with 62.3%; 14.5% of the patients were poorly differentiated (G3) and only 2.2% were well differentiated (G1). In 28 cases, there was no information available regarding tumor grading. The median follow-up was 543 days (range 47-2,202 days), and the only included patients were those with a follow-up of at least 3 months.
Catheter-based analysis
In total, 1,107 catheters were inserted in 138 patients, with a median insertion length of 12.7 cm (range, 5.0-26.9 cm). Thirteen catheters (1.2%) were classified as misplaced and were not included in the treatment plan. The median length within a lesion was 2.7 cm (range, 0.0-15.1 cm). 334 catheter tips (30.2%) were placed beyond the distal rim of the lesion. The majority (80.4%) of all catheters was inserted via CT-guidance.
Sixteen needle track lesions in close proximity to 17 catheter paths were identified, with an incidence of 1.5% per catheter placement (0.2% extra-hepatic, 1.3% intra-hepatic). Two catheters were located almost parallelly; therefore, a definite association of the seeding to one of the catheters was not possible.
Lesion-based analysis
We treated 472 liver lesions, with a mean diameter of 3.9 cm (median, 3.3 cm, range, 0.5-15.2 cm). The majority of the lesions was treated using brachytherapy with CT-guidance (67.2%). A mean radiation dose of 18.3 Gy was applied, with a maximum of 30.1 Gy. In 225 lesions (47.7%), the catheters were placed distally from the lesion rim with an average over-penetration length of 0.48 cm. Eleven needle track lesions were located proximally of the treated liver lesion and five distally. In summary, we found a frequency of 3.4% of needle track seeding per treated liver lesion. Only two lesions were considered extra-hepatic (0.4%).
Patient-based analysis
On average, 7.96 catheters per patient were inserted. The mean in-body catheter length was 103.2 cm (range, 8-320.5 cm) as the average length of all inserted catheters within a patient. Needle track seeding was found in 15 patients (10.9%, 1.4% extra-hepatic). Seeding from one singular catheter was identified in 14 patients. In one patient, two needle track lesions from two different treatment occasions were diagnosed.
During follow-up, needle track seeding occurred at an average time of 275 days (9.2 months) with median of 251 days, ranging from 92 to 509 days.
Risk assessment
In a patient-based analysis, there was a higher risk for developing needle track seeding with a high number of interventions per patient (p = 0.009), number of treated lesions (p = 0.04), and number of catheters (p = 0.03). Additionally, the overall in-patient catheter length was significant with regard to the development of track seeding (p = 0.05). Patient age also played an important role, with significant values for age at initial diagnosis (p = 0.015) and age at the first and last interventions (p = 0.017 and p = 0.01, respectively). All parameters that presented with p ≤ 0.05 were chosen for GLMM analysis. However, in this multivariate analysis, none of these possible risk factors achieved statistical significance (Table 1). In the catheter-based multivariate analysis, tumor grading was an important influencing factor, with p = 0.035 and odds ratio of 2.974. However, 243 catheters were not included in this analysis due to missing data on grading, including four catheters responsible for needle track seeding. Age at initial diagnosis and at the first intervention seemed to play a key role (p = 0.04); although statistical significance in the multivariate analysis was narrowly missed, with p = 0.07 (odds ratio [OR] = 1.05) and p = 0.07 (OR = 1.05). Another influencing factor was the guidance system, with p = 0.035 in the multivariate analysis (CT vs. MRI guidance) (OR = 2.93). Sex (p = 0.19), lesion form (p = 0.16), and over-penetration during placement of the catheters (p = 0.31) were not significant.
Discussion
In this study, we investigated the frequency of extra- and intra-hepatic needle track seeding after treatment of colorectal liver metastases by interstitial HDR-BT without application of track irradiation. We analyzed the catheter, lesion, and patient adjusted occurrence, and identified possible risk factors. As a novel approach, we were able to confidently identify needle track seeding by application of Amira® fusion software.
Extra-hepatic and especially intra-hepatic needle track seeding caused by insertion of treatment probes during local ablation of colorectal metastases, has rarely been studied [23]. However, the development of extra-hepatic seeding after diagnostic biopsies is known, especially in patients with HCC.
We identified 16 needle track lesions, 11 of which were located proximally and 5 distally of the treated metastasis. Two track lesions were located extra-hepatically.
In a study comparing brachytherapy (HDR-BT) with chemo-embolization in patients with HCC, no track seeding was identified [22]. In contrast, a study on needle track seeding after treatment of HCC with HDR-BT, reported a frequency of 1.5% per inserted catheter and 9.0% in a patient-based analysis [21].
Studies on diagnostic biopsies for HCC identified extra-hepatic frequencies of 0.8% to 3.2% in patient-based analyses [15, 18, 24, 25]. In comparison, studies on patients with colorectal cancer showed higher frequencies of needle track seeding, with 10% in a study on 51 patients [13] and even 16% in a review from 2003 [23] as well as 19% in a 2005 literature research [14]. Similar to HDR-BT, local ablative therapies, such as RFA and MWA, include image-guided insertion of a probe into a metastatic lesion. So far, there are only a few studies on needle track seeding in local ablative treatments. For RFA, there has been a reported frequency of 0% to 5.6% [20, 24, 26], and even up to 12.5% [27] in patients with HCC. A study on needle track seeding after MWA for treatment of liver tumors, reported a frequency of 0.75% [11]. A multicenter study reported a frequency of 0.15% in patients with colorectal carcinoma treated with RFA. Additionally, there are a few case reports with reported extra-hepatic seeding after RFA in patients with colorectal cancer [10, 28, 29]. All the above-mentioned studies included extra-hepatic seeding only. Evaluating possible intra-hepatic seeding is challenging, as track seeding could be confounded with an independent development of new metastases. As a new approach, we used a fusion software to match suspected track lesions with catheter paths from CT or MRI treatment plan images. Therefore, it was possible to identify intra-hepatic track seeding more confidently.
We found a patient-based frequency of needle track seeding of 10.9%, which matches previously compiled results for track seeding after biopsy of colorectal metastases. This frequency is higher than the reported frequencies in RFA and MWA in patients with HCC and colorectal metastases, which however, only considered extra-hepatic seeding.
With HDR-BT, there is no limitation regarding tumor size. With lesion sizes up to 15.2 cm, the application of multiple catheters is necessary. As suspected [20], there was a higher cumulative risk for developing needle track seeding with a higher number of catheters. However, when treating larger lesions with MWA or RFA, multiple probes or positions are needed as well. We calculated a lesion-based risk of 3.4% and a catheter-based risk of 1.5%. There seemed to be a tendency for a higher risk for developing track seeding with progressed age at initial diagnosis and age at first intervention. This could be related to an increasingly impaired immune system in older patients [30]. However, there are no similar findings to date, and this finding could also be based on probability of error.
In addition, tumor grading seemed to play an important role. Tumor cells distinguish themselves from normal tissue among other morphologic changes through decreased adhesion forces [31], thus more easily migrating to other locations. It has been demonstrated that patients with poorly differentiated primary tumors have a higher probability of developing lymph node metastases [32]. Therefore, it can be suspected that those patients are at a higher risk for track seeding. A correlation between the likelihood of track seeding after probe insertion and grading has been previously described [25, 27]. In contrast, Stigliano et al. found no significant correlation [24].
Extra-hepatic and intra-hepatic seeding
Similar to a study on track seeding after treatment with HDR-BT in patients with HCC [21], we found a low frequency of 0.2% for extra-hepatic seeding in the catheter-based analysis, 0.4% in the lesion-based analysis, and 1.4% in the patient-based analysis. These results for extra-hepatic seeding are comparable to the reported needle track seeding in RFA and MWA. In contrast, intra-hepatic needle track seeding was more frequent, with 9.5% in the patient-based analysis (3.0% lesion-based, 1.3% catheter-based). This could be explained with a usually longer intra-hepatic catheter insertion depth compared to extra-hepatic pathway, and thus higher likelihood of shedding metastatic cells. This is supported by a correlation of developing track seeding, with longer in-patient catheter length in our study. However, sufficient data for comparison on intra-hepatic seeding does not exist.
Proximal and distal location
We identified 11 track lesions located proximally of the treated liver metastases (68.8%) and five distally located track lesions. The development of track lesions located distally from the treated hepatic metastasis was presumably caused by significant over-penetration of the targeted lesion. Similar to intra-hepatic seeding in general, there are no reported data on track seeding distal from a treated lesion. It can be suspected that in a softer liver tissue as well as when treating smaller lesions, the risk of critical over-penetration is higher, as Seldinger’s technique requires an exchange of needle and catheter via angiographic wire, which might pose challenges in keeping the intra-hepatic target position. Smaller lesions might also require more frequent repositioning of the needle. Due to impaired visibility in unenhanced CT images, smaller lesions were usually treated using MRI-guidance. This could be an explanation for the higher risk of track seeding after MRI-guided interventions.
Limitations
This was a retrospective study; however, patients and treatment-related data were managed prospectively. The reasons for distal track seeding can only be suspected, as we did not obtain information on needle manipulations. A higher number of tumor passes would possibly influence the general frequency of track seeding. However, in our experience, needle misplacements usually penetrate normal liver tissue surrounding target lesion without the risk of seeding, and believed tumor penetration is followed by the insertion of a brachytherapy catheter to minimize the risk of seeding, even if a second catheter for optimal therapy results has to be inserted. Moreover, over-penetration did not seem to be a risk factor in our analysis. Additionally, data on systemic treatment following brachytherapy was not consistently available. However, systemic chemotherapy could affect the formation of track seeding after brachytherapy. Moreover, missing data regarding tumor grading worsen the assessment of grading as an influential factor. Classification and definition of track seeding and independently developed new metastases is challenging. A fusion software minimizes the risk of underestimating track seeding, but overestimation is still a possibility. Also, rigid image registration might pose challenges to correctly identify track seeding due to tissue shrinkage.
Finally, there are no sufficient data in the literature on intra-hepatic seeding, which impairs comparability to other studies.
Conclusions
Brachytherapy for treatment of colorectal metastases is associated with a similar risk for extra-hepatic track seeding compared to RFA. Including intra-hepatic seeding, which represents the majority of detected lesions, the seeding frequency is higher and similar to track seeding after biopsy of colorectal metastases. Possible risk factors could be tumor grading, using MRI guidance, and patients’ older age. After completion of the study, we implemented track irradiation, with a mean dose of 10 Gy to further reduce the risk of seeding.