The North Europe brachytherapy user meeting organized by Elekta was held on October 10-11, 2019 in London, UK. More than 80 radiation and clinical oncologists, medical physicists, and radiotherapy technologists from the UK, Ireland, The Netherlands, Denmark, Sweden, Norway, and Germany attended this meeting. Nineteen brachytherapy users from the UK, Ireland and Germany shared their experience in gynecological, prostate, skin, liver, and esophageal brachytherapy. The user meeting included a practical workshop, where users were given the opportunity to obtain hands-on experience with both treatment planning software and applicators.
Gynecological brachytherapy
Most of the presentations were dedicated to gynecological brachytherapy. Pauline Humphrey from the Bristol Hematology and Oncology Centre reported results of a UK survey with the goal to obtain insight in the brachytherapy practice for locally advanced cervical cancer. The online survey consisted of questions about brachytherapy scheduling, inpatient/day case treatment, anesthetic/analgesic protocols, and non-pharmacological support. Responses were received from 39/43 eligible centers. Brachytherapy was predominantly given on an inpatient basis in 65% and as day case treatment in at 35% of centers. Eleven different brachytherapy scheduling regimes were reported. The typical duration of brachytherapy at each center (number of hours with applicators in place per insertion) varied from 3 to 53 hours. Generally, the respondents were positive about the level of care and assistance offered and provided many examples of good practice but also suggestions on how to improve the patient pathway. Free text answers were given by 33 respondents to the question “What works well in your department?”. Three main categories were identified and included “continuity of experienced staff”, “good information and support”, and “trust and rapport”. The question “What could be improved?” was answered by 32 respondents, and three main categories were identified such as “follow up provision”, “pain relief”, and “care in hospital wards”.
Results from another UK high-dose-rate (HDR) cervix brachytherapy survey were presented by Claire Fletcher from the University Hospitals Coventry and Warwickshire NHS Trust. In 2018, the HDR cervix brachytherapy service at Coventry was being reviewed due to the number of sessions where treatments occurred out of hours. In June 2018, a questionnaire was sent out to the medical physics e-mail forum to determine HDR service provision in UK centers. 19 centers responded with a wide range of different activity levels. In September 2019, centers were contacted to see if any aspect of their service had changed.
The main findings of the survey were as follows: 79% of all centers who responded were planning cervix brachytherapy conformally and almost all centers had at least some access to MRI. Nearly 60% of centers were using interstitial brachytherapy with most of insertions being performed under ultrasound guidance. The majority of centers were using 3 fractions in a treatment, with each fraction given on a different insertion. However, 47% of centers who responded were applying multiple fractions on a single insertion, and these centers were more likely to give 4 fractions (e.g. 2 insertions with two fractions each). Most centers inserted applicators under general anesthesia, but using a spinal block could speed up the procedures’ time and finish the treatment before 6 p.m. One third of all responding centers, routinely or regularly were treating patients outside the usual working hours, but there was a large variation in finishing times even within one center. The greater the complexity of treatment, the higher the chance that the procedure days will run late. However, there were also many centers that used conformal planning and did not routinely proceed with treatment out of routine hours. 63% of all centers had more than one dedicated planning computer, but it did not seem to have a large correlation with finishing time. When the questionnaire was reviewed in 2019, two centers were finishing earlier than previously reported. The reasons suggested included staff availability and training as well as implementation of automated checking procedures.
Jennifer Cannon from the James Cook University Hospital shared preliminary results of a UK audit of cervix brachytherapy treatments. Computed tomography (CT) and magnetic resonance (MR) images of two patients previously treated with brachytherapy were distributed to eleven UK radiotherapy centers. The patients were selected to represent a typical patient cohort: an intracavitary treatment only, and a combined intracavitary and interstitial treatment. Centers were asked to delineate target volumes and produce treatment plans on a standard set of delineations, following their local protocol. The variation in target volume delineation was assessed in terms of absolute volume variation, and dose optimization was evaluated with regard to dose-volume histogram (DVH) statistics and total EQD2. A further assessment was made by estimating the parameters based on all centers’ submissions and comparing them to submissions from centers that specifically followed the EMBRACE II protocol. A variation in interstitial techniques was also assessed by comparing parameters such as total TRAK, needle TRAK, and maximum needle dwell time. This work is currently under Journals’ review and results from this study will be published soon.
A presentation of Rhydian Caines from the Clatterbridge Cancer Centre NHS Foundation Trust was dedicated to prescription to protocol for HDR cervix brachytherapy. HDR image-guided brachytherapy for cervix cancer is administered in this center following a three-fraction day case model as per the EMBRACE II protocol. CT/MR image fusion is used for each fraction individually planned on Oncentra planning workstations, and delivered on the same day with a Flexitron HDR afterloader. With over 150 treatments delivered per year, the local team is very experienced, but clinician availability at the end of the process for final plan approval is often limited due to multiple competing responsibilities, resulting in additional wait time for patients. A preliminary audit of 92 fractions indicated that a mean interval between plan check and clinician approval was around 14 minutes; however, in 22% of cases, the time was exceeded by 20 minutes and in a few cases, even by 70-100 minutes. Prescription to protocol (PP) is a framework, under which a treatment plan with pre-agreed dose constraints is authorized for a treatment by an appropriately trained and experienced medical physics expert (MPE), instead of a clinician. Justification of the exposure is established by a clinician through authorization of the protocol. A preliminary review of 69 previous fractions suggested that the EMBRACE II optimal planning aims, when radio-biologically transformed into single fraction equivalents, provided an appropriate basis for this protocol, with around 20% of plans meeting all criteria (Table 1).
Table 1
The EMBRACE II single fraction constraints adopted for prescription to protocol (PP) implementation for IGBT of cervix cancer D90
| Parameter | Constraint (Gy) |
|---|---|
| HR-CTV D90 | < 10.1 |
| HR-CTV D90 | > 9.4 |
| HR-CTV D98 | > 7.2 |
| GTV res D98 | > 9.4 |
| Point A mean dose | > 5.4 |
| Rectum D2cc | < 4.6 |
| Bladder D2cc | < 6.4 |
| Sigmoid D2cc | < 5.2 |
| Bowel D2cc | < 5.2 |
| Recto-vaginal point | < 4.6 |
In six months following implementation, 12 of 87 patents were authorized for treatment under PP, with 8 of 29 patients having at least one MPE authorization. The main failure mode was point A, which among the 75 ineligible plans had failed 43 times (under local policy, more weight is usually given to the HR-CTV D90 upper limit). Median time from plan check to plan approval was 3 (0-13) minutes for MPE compared with 11 (1-48) minutes for a clinician (p < 0.01, Mann-Whitney); however, this did not statistically influence the total patient wait time.
In conclusion, early experience with PP has been encouraging, with fewer handovers, streamlined workflow, and a reduced clinician burden. A significantly reduced wait time for plan approval was demonstrated. Low A point dose was the most common failure mode due to the local tendency to favor a lower HR-CTV D90 (< 10.1 Gy).
Nancy Lewis from the University Hospital Southampton NHS Foundation Trust reported experience with Elekta’s advanced gynecological applicator Venezia. This hybrid applicator allows the combination of intracavitary brachytherapy with interstitial brachytherapy. The local cervix cancer treatment protocol included EBRT of 45 Gy in 25 fractions and HDR brachytherapy consisting of 26 Gy in 4 fractions, with brachytherapy given using two applicator insertions, 1 week apart. All insertions were performed under spinal anesthetic, with interstitial patients receiving an epidural + PCA. Patients selected for possible interstitial treatments received an MRI in week 5 of external beam treatment. From the operating room, patients were taken for MRI and CT imaging.
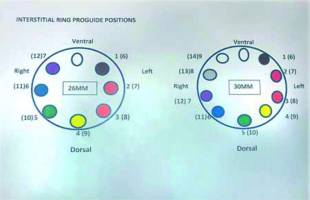
Since March 2018, seven patients were treated with the hybrid Venezia applicator: 3 patients with the standard tandem and ovoids configuration in combination with a template and needles, 3 with the standard configuration in combination with oblique needles, and one patient with the standard combination using a 70 mm intra-uterine tube. In case of interstitial treatment only sharp needles were used. The sizes of available ovoids were 26 mm and 30 mm. Vaginal caps were not used. Three patients treated with a template and needles had an anterior vaginal extension. Oblique needles were used when the high-risk clinical target volume (HR-CTV) was too wide, to be adequately covered with parallel needles. Insertion of oblique needles was similar to the insertion of parallel needles, only slightly more pressure was required. Positions of needles were changed between implants in only one patient. Needle could be positioned in the expected positions as confirmed using CT imaging. Needle positions were reproducible. Conclusion was that the Venezia applicator allows for treatment of tumors that would otherwise be referred elsewhere. Careful consideration should be given to vaginal packing and wise choice of needles as well as to emergency procedures.
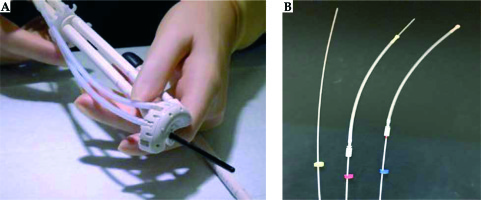
Similarly, Bettany Cushing from the Mater Private Hospital in Dublin (department led by Dr. Michael Maher) shared her experience with the advanced gynecological applicator Venezia. Patients that were selected for treatment with the Venezia applicator usually receive EBRT consisting of 50.4 Gy in 28 fractions. They have a pre-brachytherapy MRI after 20 EBRT fractions (36 Gy) to determine the size of applicator to be inserted for optimal fit. HDR brachytherapy fractionation consists of 21 Gy in 3 separate insertions. The brachytherapy procedure is as follows: the patient is admitted into the department the day before the treatment, fasting from midnight. A radiation therapist (RT) delivers the brachytherapy equipment to an operating theatre the night before the procedure. This brachytherapy equipment includes a cervix set (two ovoid tubes that together form a ring, and an intrauterine (IU) tube), a rectal retractor, 2 rubber bungs, guiding tubes, ProGuide needles, and color-coded cable ties. All patients receive an epidural anesthesia, and have a urinary catheter placed. The brachytherapy applicators are inserted under general anesthesia. The insertion is ultrasound-guided or by feel through the consultant’s experience. The cervical ring is preloaded with needles, which are secured in position with guiding tubes. The IU tube is inserted into the intrauterine canal and the ring is positioned at the neck of the cervix. The rectal retractor pushes the rectum away and the needles are advanced through the ring using an insertion tool. Packing such as wet gauze is used to secure the entire application in position. The position of each ProGuide needle using color-coded cable ties is recorded, so that the exact position of each needle can be tracked, and each needle can be connected to the Flexitron in the proper order before treatment delivery (Figures 1, 2). Adherence to this protocol eliminates the risk of needles being connected to the incorrect channels of the Flexitron.
When interstitial needles are used, the patient requires both an MRI and CT-scan for planning purposes. This is in contrast with implants without needles, where only an MRI is necessary. As for the MRI, marking of the needle positions with a permanent marker is performed as well as removing the steel obturators from the ProGuide needles. The function of the MRI is to ensure the IU tube is in correct position. With respect to the CT, gold marker wires are placed in the ProGuide needles to verify their position, and specific marker wires are placed into the ring and the IU tube.
The physics team carries out the planning, which is then assessed and signed off by the radiation oncologist. The pretreatment record is then presented to the brachytherapy radiation therapy team and verified with the Oncentra brachytherapy plan. This is to ensure the compatibility of the prescription, indexer length, dwell positions, and treatment times as well as the immobility of ProGuide needles. Transfer tubes 1 + 3 are placed in channels 1 + 5. ProGuide needles are connected as per the diagram on the treatment sheet. Once the treatment is delivered and completed, before exiting the room, it is insured that the source has retracted completely and the wall monitor outside and inside the room show “zero” on a Geiger counter. Check time on post-treatment record should be equal to the time on pretreatment record. The consultant can subsequently remove the urinary catheter and applicators. There is limited number of research papers to serve as a comparison for future studies including patients with two brachytherapy treatment plans created, one with needles and one without to demonstrate the advantages of using interstitial needles. It is expected that the outcome of such a comparison would demonstrate higher dose delivery to CTV, while maintaining dose-volume constraints for organs at risk.
Claudia Hill from the University Hospital Southampton NHS Foundation Trust elaborated on the use of inverse planning for multi-channel vaginal HDR brachytherapy. The purpose of this project was to compare the current protocol, using manual and graphical optimization and two established inverse planning methods: inverse planning simulated annealing (IPSA) and hybrid inverse treatment planning and optimization (HIPO). For 13 gynecological cancer patients previously treated with HDR VMC brachytherapy, a planner retrospectively reoptimized the dose distributions using three different methods: manual and graphical optimization, IPSA, and HIPO. Relevant dose-volume histogram (DVH) parameters (HDvol V100 and D90, LDvol V100 and D90, rectum D2cc, bladder D2cc) were recorded in addition to the dose homogeneity index, central catheter loading index, and treatment parameters such as the number of dwell positions and total dwell times. Also, treatment planning time was evaluated. Both inverse methods resulted in shorter planning times as compared to manual and graphical optimization. In terms of DVH parameters, inversely optimized plans were comparable to the manually optimized plans. There was no statistical significance between the quality of differently optimized plans. In conclusion, inverse planning methods decrease the planning time as compared to a combination of manual and graphical optimization, and the DVH statistics are comparable with all methods. HIPO is the most suitable inverse optimizer for VMC brachytherapy due to the ability of the operator to control activated dwells.
Michael Niekamp from Elekta presented advanced brachytherapy planning tools for interstitial cervix applications: IPSA, HIPO, and implant modeling. He emphasized that the treatment possibilities with the new hybrid gynecological applicators are increasing. Nowadays, the variation of standard-shaped applicators in combinations with additional interstitial needles create real 3D volume implants. Furthermore, the number of combinations of dwell times and their positions is uncountable. He presented intelligent tools, which help to establish new adapted brachytherapy workflows:
Predefined protocols for contouring, prescription, dose optimization, and evaluation lead to standards procedures.
Applicator models with 3D source paths will accelerate dose planning, resulting in more reproducible and accurate doses.
Intelligent tools for dose optimization and real-time inverse planning help to decide the best adapted implant geometry and optimal dose distribution for treatment. The automation will reduce the overall planning procedure with full control by the user at any time.
Specific workflows with the right utilization of the presented advanced brachytherapy tools will lead to an improvement in treatments.
In the second presentation, Michael Niekamp shared his thoughts about the integration of Elekta Brachytherapy in the Oncology Information System (OIS) for radiation oncology. In medicine, there is no doubt that brachytherapy as a complementary treatment, improves tumor control, increases overall survival, reduces side effects, and improves the quality of life after treatment. Brachytherapy needs to be an integral part of cancer treatment, especially in radiation therapy. The presentation showed how an open architecture of various digital tools and their special tasks are adapted for the user (e.g., patient management, diagnostics, targeting, treatment planning and delivery), which optimizes the patient’s way through a radiation therapy department. The idea presented in this session is not limited to the integration of brachytherapy treatment execution alone. With a clever assessment and analysis of existing tasks and workflows, one can optimize workplaces by using recent excellent, well-implemented, and accepted functions across all treatment modalities that are required to cure patients. Possibilities and solutions for different platforms provider were discussed.
Christopher Lee from the Clatterbridge Cancer Centre NHS Foundation Trust reported results of paperless integration of Elekta’s brachytherapy planning and treatment delivery system within a 3rd party record and verification procedure for HDR image-guided brachytherapy of the cervix. At the Clatterbridge Cancer Centre, paperless workflows through the considered use of various management tools available within ARIA 13.6 (Varian Medical Systems, CA) were introduced for Image-Guided Brachytherapy (IGBT) for cervical cancer. This enabled integration of brachytherapy treatment planning (Oncentra Brachy) and treatment delivery system (Flexitron HDR) within ARIA.
The department delivers approximately 150 fractions per year, in two treatment sessions per week. Each fraction uses CT/MRI image fusion with treatments individually planned on an Oncentra Brachy workstation and delivered the same day using a Flexitron HDR afterloader (Elekta, Sweden). The work is complex, very time-sensitive, with several handover points, and critically depending on good communication between staff groups within the team.
Following a review of the previous paper-based process, a new paperless workflow was designed by a multidisciplinary brachytherapy team involving radiographers, physicists, and clinicians. The new paperless workflow clearly emphasizes the safety and efficiency of treatment, with clear communication of salient clinical information. Several ARIA’s key workspaces namely electronic prescription, care path workflow management, and documents are utilized. Sign off and approval functions within ARIA serve as definitive legal signatures and provide an audit trail for the different tasks and responsibilities within the procedure. Following implementation, a timing audit was carried out to compare the duration between imaging and treatment for both the paper and paperless procedures. This showed that the overall time was similar for the two processes, but more patients on average were treated with the paperless procedure.
The implementation of paperless working within IGBT for cervix cancer had a positive effect on efficiency for this highly time-sensitive clinical process. Despite treating significantly more patients, waiting times have not increased and all treatments were concluded comfortably within clinical working hours. The brachytherapy team has embraced the changes, which benefited the patients. Electronic workflow management allows easy collection of rich audit data to leverage and measure efficiencies of the pathway and determine areas for future optimization.
Robert Dacey from the Northern Centre for Cancer Care (NCCC), Freeman Hospital Newcastle upon Tyne presented benefits of automated brachytherapy planning checks using the DICOM RT plan file. With increasingly complex means of producing and delivering dose distributions, the number of parameters having an impact on treatment delivery is continually expanding. Independent checking of plan parameters to avoid errors in treatment delivery is a well-established practice in radiotherapy; however, the focus has primarily been on external beam deliveries with brachytherapy checks concentrating mainly on dosimetric aspects alone.
Until recently, 1-2 patients were treated at the NCCC each week. Before introducing the use of interstitial needles into routine clinical practice (approximately 18 months ago), a timing study was undertaken to assess the possibility of increasing overall efficiency while maintaining safety in anticipation of a greater workload (3 cases per week are now a routine). Checking plan was identified as one part of the process taking a significant period of time, with the previous “tick box” check-form requiring visual assessment of 10-100 s of parameters, making it both time-consuming and prone to error. It was therefore decided to investigate the possibility of automating the extraction and manipulation of data directly from the DICOM RT plan file (as exported from Oncentra Brachy) to provide a rapid, accurate, and consistent method of checking.
A template was constructed in Microsoft Excel and a series of scripts written in Microsoft Visual Basic, where scripts guide the user through the process and prompt for details where necessary. Before data can be extracted from the DICOM RT plan file (in plain text), it must be filtered to remove extraneous DICOM formatting. The majority of parameters may be identified by unique DICOM tags (e.g., patient name, ring diameter, plan ID); however, some are associated with a common tag to multiple DICOM items (e.g., indexer lengths, channel ID) that must be dealt systematically. Several parameters of interest are not explicitly declared and must be determined via calculation involving some individual DICOM items (e.g., dwell times) including assessments of geometry. The latter is best performed in the applicator frame of reference requiring a linear transformation of the patient frame of reference co-ordinates from the DICOM RT plan file. Extracted values are transformed to the Excel template, where they are applied in calculations or compared directly to reference values.
Therefore, a method has been identified, where with minimal user input, a significant number of checks of the clinical brachytherapy plan are performed in a matter of seconds, thus significantly increasing the speed of checking without compromising on consistency or accuracy.
Skin brachytherapy
Skin brachytherapy was one of the main focus areas of the meeting. Agata Rembielak from The Christie NHS Foundation Trust and The University of Manchester shared an overview of recently published GEC-ESTRO ACROP guidelines and recommendations for skin brachytherapy. The guidelines constitute a unique publication in skin brachytherapy and provide eight main conclusions with main remark stating that “no standard schedule can be recommended, and total doses are based on experience”. It was also advised that “the dose on the skin surface should be recorded to correlate the outcome with late side effects”. The full document is available as free access text at https://www.thegreenjournal.com/article/S0167-8140(18)30035-5/pdf (source: Guinot et al. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol 2018; 126: 377-385, DOI: https://doi.org/10.1016/j.radonc.2018.01.013).
In the second part, Agata Rembielak elaborated on the history of skin brachytherapy at The Christie, where clinical applications started shortly after radium discovery in 1898, and has been provided as an uninterrupted clinical service until present days. Agata’s talk was supported by pictures from The Christie archive illustrating radium applicators and applications in patients. She described the evolution of skin brachytherapy from radium and manual positioning to automated remote afterloading technique. The main part of her talk was dedicated to the experience of The Christie team, with the so-called Christie method of skin brachytherapy. In this method, skin brachytherapy is delivered using a handmade mould or flap applicator, but with an extra layer of a bolus of various thickness, depending on clinical situations. The talk was supported by clinical examples demonstrating excellent cosmetic outcomes and low toxicity from skin brachytherapy, particularly in challenging locations such as face, hands, fingers, legs and feet.
Orla Brosnan from the Mater Private Hospital in Dublin, reported results of a treatment of skin lesions on the head using thermoplastic moulds in conjunction with the Freiburg flap. Due to spherical shape of the target volume, it is hard to achieve a homogenous dose distribution using EBRT. It is also difficult to obtain a flush finish using the Freiburg flap and to eliminate air gaps on the surface of the head if lesions are raised or irregularly shaped. This led to investigating customized mould-based surface brachytherapy, with an HDR afterloading procedure frequently used in other parts of the body. A silicone rubber applicator material (the Freiburg flap) is attached to a customized thermoplastic mould shaped to the patient’s head. When non-invasive topical therapies failed or the lesion returned, treatment options for large lesions, multiple lesions, and lesions at difficult sites like the scalp are limited. Surgery for these patients results in poor wound healing rates and can be cosmetically unacceptable. Radiation therapy is a valid treatment option for these patients, with good local control and good cosmetic outcome.
The inclusion criteria for mould-based brachytherapy involve superficial small and large-sized lesions as well as angiosarcoma, squamous cell carcinoma (SCC), and basal cell carcinoma (BCC). It is particularly useful for elderly, frail patients who may not be able to lie flat. It is also suitable for patients using blood thinners, or in sites at risk for delayed healing with surgery. It has a potential use for melanomas and Merkel Cell tumors, depending on clinical assessment. Exclusion criteria involve lesions on the inner and outer canthus, lesions with perineural extension, and recurrent disease with possible deep extension to the bone.
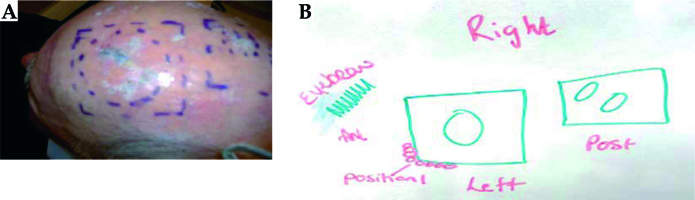
Pretreatment moulding procedure: the consultant defines the treatment area on the patient’s skin using a marker pen and the radiation therapist draws the treatment area (Figure 3) – angiosarcoma.
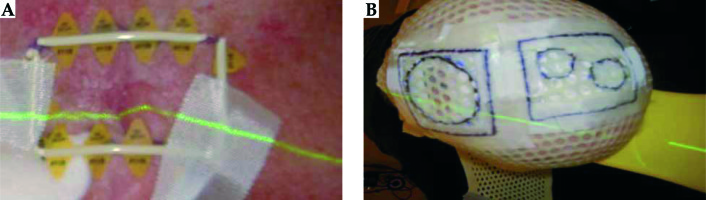
Fiducial marker wire is placed on the skin outline (Figure 4A). The tape is placed over the fiducial marker and the fiducials are traced with a permanent marker, so that it is visible under the thermoplastic material. The water bath is turned on approximately 1.5 hours before the procedure. The thermoplastic material is cut to the required size. The patient is in a supine position with the head in an appropriate headrest. The thermoplastic mould is placed in the water bath for 2.5 minutes to soften the plastic. It is then molded onto the patient head.
The thermoplastic material takes a minimum of 10 minutes to harden. Before it is removed, a tape is placed over visible fiducial markers and the treatment area is drawn on the mould (Figure 4B). Fiducial markers on the patient’s skin should correspond to the marks placed on the thermoplastic material. The patient goes home and returns at a later date for CT planning.
Pretreatment attachment of the Freiburg flap: the appropriate size and number of Freiburg flaps channels are selected. One treatment position is determined for all areas. The optimal configuration of the flap is determined to ensure it is as flush as possible to the thermoplastic material. The flap is sutured roughly in place using dental floss. Sutures should never be running across the catheter, but rather running lengthways down the beads to avoid obstruction of the source.
Planning CT procedure: the treatment area was drawn on the patient’s skin and the fiducial marker was placed onto the area. The thermoplastic material was fitted on the patient, ensuring that it was flush with the skin. CT markers were inserted into appropriate number of channels on the flap (i.e., equal to the width of field, which was marked on the skin). Sandbags were positioned on top of the flap to ensure that there were no air gaps between the thermoplastic material and the skin. CT scan (3 mm slices) was performed and the scans were sent to Prosoma for planning.
The planning image (Figure 5) shows a 100% dose distribution to the skin surface. The prescribed dose was 36-40 Gy in 10 fractions, delivered twice per week, over 5 consecutive weeks. The patient’s treatment position should be determined during first fraction. The patient can sit up if necessary. Planned number of transfer tubes was attached to the Flexitron. The thermoplastic material was placed on the patient and transfer tubes were connected to the Freiburg flap. Sandbags should be used to eliminate air gaps. The radiation therapists completed the pretreatment checks and the treatment was started.
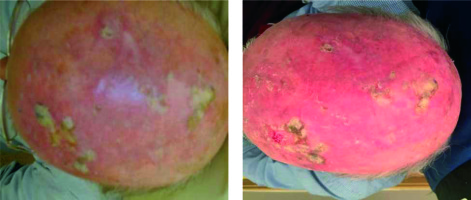
To date, four patients have been treated, all with good cosmetic outcomes (Figure 6). After treatment all patients had increased social interactions, resulting from a more positive body image and less self-consciousness.
Satiavani Ramasamy from the St. James University Hospital reported their initial clinical outcomes obtained with basal cell carcinoma treatment with the Elekta’s Valencia applicator. The Leeds Cancer Centre established a skin brachytherapy service using Valencia applicators in September 2017. They audited and performed quality assurance of initial experience and effectiveness of outcomes; all patients were treated between September 2017 and August 2019. The dose used was 42 Gy in 6-7 fractions twice a week (biologically effective dose [BED] equivalent 70 Gy). For quality assurance, a multi-disciplinary team consisting of physicists, clinician-scientists, oncologists, mould room technicians, and radiographers analyzes each brachytherapy case before treatment initiation. Patients were followed up and reviewed in 8-10 weeks after treatment completion. 41 patients were included in this study, with a median follow-up of 4.7 months (range, 1.1-24.4 months). The median age was 73 years (range, 55-92 years). WHO performance status was 0 (88%, n = 36), 1 (10%, n = 1), and 2 (10%, n = 1). A total of 43 lesions were treated with a mean size of 1 cm (range, 0.2-2 cm). All were basal cell carcinoma. Sites of lesions were cheek (14%, n = 6), chin (2%, n = 1), ear (2%, n = 1), nose (37%, n = 16), lips (14%, n = 6), scalp (7%, n = 3), forehead (21%, n = 9), and extremities (2%, n = 1). The reason for choosing skin brachytherapy was driven by patient preference in 36 patients.
All patients completed treatment without any delay. 5 patients (6 lesions) have not had 2 months follow-up so far. Out of the 36 patients (37 lesions), with a minimum of 23 months follow-up, 100% had a complete clinical response at 8-10 weeks post-treatment. Treatment was well tolerated with no unexpected toxicities. No recurrences were identified in the cohort until now.
HDR brachytherapy for NMSC is effective, safe, and very well tolerated. Support from the multidisciplinary team was essential for the successful implementation of the service. The Valencia brachytherapy applicator allows the delivery of hypofractionated radical radiotherapy as an option for skin lesions up to 20 mm in size and 3 mm in depth.
Other indications
Mark Long from the St. Luke’s Cancer Centre in the Royal Surrey County Hospital reported results of implementation and clinical experience of HDR prostate brachytherapy in Royal Surrey. The hospital provides a broad range of brachytherapy services, including Papillon rectal electronic brachytherapy, image-guided gynecological HDR brachytherapy treatments, and a well-established “4D brachytherapy” LDR prostate technique. The center wanted to expand the service to be able to offer T3 prostate cancer patients with an option of HDR prostate treatments. Oncentra Prostate was purchased and commissioned for this purpose. The US-guided planning and treatment process was developed to align with the experience from the center’s other brachytherapy services. Blending the process used from LDR treatments allowed to sidestep the virtual contouring phase and develop own pseudo-virtual planning phase with the intent to reduce the time of procedure.
Since May 2018, 21 patients have been treated. It is hoped that with more experience, close communication with the brachytherapy community, and with auditing, the technique will be further developed and become more efficient.
Another presentation about prostate brachytherapy was given by Katie McHugh from the Cambridge University Hospitals NHS Foundation Trust who shared clinical data from Addenbrooke’s Hospital, where over 750 patients have been treated using LDR prostate seed implants using the Nucletron/Elekta system since 2006 (Oncentra for planning, and SeedSelectron for delivery). All patients have a post-CT scan at 6 weeks after the treatment, and their post-implant dosimetry is calculated and compared with the published RCR guidelines for a satisfactory implant. Towards the end of 2018, the hospital was given an end of life notice for the supply of seeds that fit the Seed Selectron for May 2019, so they had just over 5 months to set up and commission a new system while maintaining a clinical service. They opted to keep the current TPS and found a new seed supplier who provided seeds to fit in the Mick applicator, which also had to be purchased and commissioned. Commissioning involved updating TPS seed model, setting up a method of seed QA, revising contingency plans and risk assessments, and staff training. Up to September 2019, 26 patients were treated, with no break in service delivery and with no significant changes in the hospitals’ live plan stats or post-implant dosimetry. The hospital made several service improvements and can now treat patients with larger prostates, reduce operating time, and have a cost saving of up to £35k per year.
Stefanie Corradini from the Hospital of the Ludwig-Maximilians University (LMU) in Munich shared her experience in CT-guided interstitial brachytherapy of liver malignancies. Brachytherapy (BT) is an option for the treatment of liver-malignancies in patients with primary liver malignancies (e.g., hepatocellular carcinoma, cholangiocellular carcinoma) or patients with oligometastatic disease. Although brachytherapy is a long-standing, minimally invasive treatment method, it has not been widely implemented for an ablation of liver lesions. Nevertheless, there is emerging evidence for the effectiveness of this method of treatment, and the technique has recently been added to the “toolbox of ablative treatment options” of the European Society for Medical Oncology (ESMO) guidelines for metastatic colorectal carcinoma and hepatocellular carcinomas. Brachytherapy is known to achieve comparable results to SBRT and radiofrequency ablation (RFA) in the treatment of liver malignancies, with excellent local tumor control rates. Brachytherapy is preferred over RFA for large lesions (> 3 cm), in the proximity of large vessels (no cooling effect) or irregular tumor shape, or as an alternative to SBRT in the proximity of organs at risk (e.g., stomach, bowel), and for very large or multiple lesions.
At LMU Munich, the procedure is performed in close collaboration with the department of interventional radiology. The workflow is as follows: first, diagnostic imaging with hepatospecific contrast-enhanced MRI (Primovist) is acquired for staging and all cases are discussed in a multidisciplinary tumor board. The placement of the brachytherapy catheters is performed by the interventional radiologist under fluoroscopy-CT guidance and local anesthesia. Midazolam and fentanyl are given for sedation and analgesia as individually required by the patients. Hollow 17-gauge needles are placed into the lesions. Thereafter, an angiography sheath with a 6F diameter is inserted over a stiff angiography guidewire, and the 6F brachytherapy catheters are consecutively placed in the angiography sheaths. In case of large lesions, multiple catheters are inserted. After the placement of catheters, a contrast-enhanced planning CT is acquired using a breath-hold technique and a slice thickness of 3 mm, which is used for treatment planning. The patient is then transferred to the brachytherapy unit. Treatment planning is performed using the Oncentra®Brachy treatment planning system (Elekta AB, Stockholm, Sweden). Target delineation consist of gross tumor volume (GTV), with an additional margin of 3-5 mm for the clinical target volume (CTV) depending on visualization quality of the GTV. Usually, there is no additional target volume planning (CTV = PTV). Moreover, organs at risk (OAR) such as liver, stomach, duodenum, colon, small intestines, and heart are delineated. After catheter reconstruction, treatment planning and dose optimization are performed. The aimed prescription dose (D100) depends on histology and varies between 1 × 15 Gy (HCC) and 1 × 20 Gy (metastases), or 1 × 25 Gy (colorectal metastases), taking into account organ at risk constraints. Moreover, the entire needle track is treated up to a dose of ~5 Gy to prevent needle track seeding.
Brachytherapy is applied using an HDR afterloading system (Flexitron, Nucletron, Elekta AB, Stockholm, Sweden) with an iridium-192 (192Ir) source. The treatment time depends on the size of the lesion, number of catheters, dwell positions, and the activity of the source, and can last up to 60 min or even longer in very large lesions. For catheter removal, a gel foam is introduced through each angiography sheath during removal to prevent bleeding.
Taken together, BT allows to treat also very large lesions with single doses of 15-25 Gy and results in excellent local control rates (> 90%) in large primary or secondary hepatic lesions of up to 12-15 cm. Moreover, in contrast to thermoablative treatment approaches (RFA), it provides an effective treatment option for centrally located liver lesions, close to large vessels. When compared to most SBRT techniques, BT is less affected by uncertainties related to respiratory breathing motion, as the tumor is fixed by the implanted catheters. Another advantage of BT is the possibility of a repetitive approach, with the application of BT using hypofractionated fractionation schedules, with two or three fractions to spare OARs or treat very large tumors. Regarding adverse events in BT, significant bleedings following the interventional catheter implantation occurred in 4% in HCC and 2.5% in colorectal liver metastases. Additionally, the risk for radiation-induced liver disease (RILD) seems to be very low, even after the treatment of very large liver tumors or in livers with underlying cirrhosis.
Joshua Mason from the St. James University Hospital presented results of a study of his colleague Luke Eason about the evaluation of a collapsed-cone convolution algorithm for 192Ir brachytherapy treatment planning for esophageal and surface mould brachytherapy treatments. The purpose of the study: TG43 does not account for a lack of scatter and tissue and applicator heterogeneities. The advanced collapsed-cone engine (ACE) algorithm available in the Oncentra Brachy (OCB) treatment planning system (Elekta AB, Stockholm, Sweden) can model these conditions more accurately and is evaluated for esophageal and surface mould brachytherapy treatments.
ACE was commissioned for use and compared against TG43 for five esophageal and five surface mould treatment plans. Dosimetric differences between each algorithm were assessed using dose difference maps, side-by-side comparisons, and DVH statistics.
Results of the study:
Esophagus (6 Gy per fraction): Compared to TG43, ACE demonstrated up to a 0.63% and 0.05 Gy reduction in PTV V100 and PTV D98, respectively. Lung D2cc and bone D2cc deviated by up to 0.09 Gy and 0.03 Gy, respectively. Lung D0.1cc and bone D0.1cc both deviated by up to 0.12 Gy. Surface mould (4.5 Gy per fraction): Compared to TG43, ACE demonstrated up to a 12.5% and 0.18 Gy reduction in PTV V80 and PTV D98, respectively. Bone D2cc and D0.1cc both reduced by up to 0.2 Gy when modeled with ACE. Increasing mould size laterally increased the dosimetric differences between TG43 and ACE.
TG43 generally overestimates the dose delivered to the target volume and OARs for the clinical sites investigated. Dosimetric differences observed for esophageal treatments were minimal; however, treatments involving larger surface moulds would benefit from increased dosimetric accuracy offered by ACE. Implementation should be considered for surface mould 192Ir treatment planning, but increased calculation time, additional contouring, and mass density assignment requirements should be scrutinized with regards to their potentially negative impact on current clinical practice.