Purpose
Interstitial brachytherapy in patients with locally advanced cervix cancer has gained attention in recent years, with the emergence of modern hybrid intra-cavitary and interstitial (IC/IS) brachytherapy applicators. Historically, common applicators for gynecological interstitial brachytherapy included Syed-Neblett template and Martinez universal perineal interstitial template (MUPIT), with transperineal insertion of interstitial needles [1, 2]. However, these approaches are highly invasive, requiring in-patient stay, and are always performed in an operating theatre under spinal, epidural, or general anesthesia. Although transperineal interstitial brachytherapy would still play a role in treating disease in the lower vagina, the necessity of skills and expertise has resulted in a decline in popularity in favor of hybrid applicators.
Hybrid applicators were developed to allow trans-vaginal needle placement within the ring or ovoids into the parametrium, but this technique requires more additional time and learning curve compared with standard intra-cavitary (IC) brachytherapy. Hybrid applicators provide multiple technical advantages; they are less operator-dependent, less invasive, and less painful to patients, and thus suitable for daily procedures. These favorable attributes can result in a reduction in anesthetist requirement and hospitalization stay with potential resource and cost savings. Moderate sedation with midazolam and/or fentanyl was prospectively reported to be safe, effective, and therefore the desired sedatives are intended for cases who are not supervised by an anesthetist [3-6]. The American Brachytherapy Society recommends that moderate sedation should be used for high-dose-rate (HDR) brachytherapy whenever possible [7]. In our institution, the routine practice for gynecological brachytherapy involves the use of moderate sedation due to limited access to anesthesia services. However, it is worth noting that the existing literature primarily focuses on the use of spinal, epidural, or general anesthesia with hybrid applicators; however, data on the use of moderate sedation are limited [8]. In January 2017, our institution introduced the IC/IS brachytherapy service to address the needs of patients with locally advanced cervical cancer. In a previous publication, specific details regarding the anesthesia aspect of moderate sedation were provided, and demonstrated the feasibility and tolerance of hybrid applicator placement under moderate sedation [6]. In the present study, our primary focus was to report on the oncologic outcome and toxicity profile of patients who underwent this approach.
Material and methods
From January 2017 to April 2021, a total of thirty-three patients with non-metastatic cervical cancer and the American Society of Anesthesiologist physical status I-II underwent sixty-nine fractions of interstitial brachytherapy using hybrid applicators in the Department of Radiation Oncology (DRO), National Cancer Centre, Singapore [9]. All patients were staged according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system [10]. Data were prospectively collected and analyzed retrospectively. All patients referred to DRO of our institution were routinely requested to sign an informed consent form to collect clinical data used for the purpose of research.
All patients were treated with external beam radiotherapy (EBRT) between 45 Gy and 50.4 Gy in 1.8 Gy per fraction, with nodal boost ranging from 3.6 to 9.0 Gy. Patients received weekly cisplatin of 40 mg/m2 up to six cycles concurrently with EBRT. Brachytherapy commenced either on the final week of EBRT or within 1 week of finishing EBRT. No chemotherapy or EBRT were allowed on the days of brachytherapy.
All patients underwent clinical examination and magnetic resonance imaging (MRI) of pelvis without applicator in situ within one week before the first session of brachytherapy, to evaluate the extent of tumor response. Clinical and radiological findings of tumor extent pre-brachytherapy would aid in determining the suitability for interstitial brachytherapy based on predicted isodose distribution of intra-cavitary brachytherapy alone. Patients were treated with a variety of modern hybrid interstitial brachytherapy using either tandem with ovoids (Utrecht applicator) or tandem with ring (interstitial ring applicator and Venezia applicator). Final choice of an applicator was determined by a combination of factors, including availability of applicators, patient anatomy, and tumor topography. Number, position, and depth of the required needles were pre-planned by a radiation oncologist, with an input from a radiologist and brachytherapy physicist.
Every patient underwent a total of three to four fractions of brachytherapy, with at least one fraction of interstitial brachytherapy, and the remaining fractions with standard intra-cavitary brachytherapy. A commonly employed fractionation schedule consisted of two fractions of interstitial brachytherapy, spaced one week apart. Patients who experienced significant tumor shrinkage at the time of second fraction of interstitial brachytherapy subsequently received one to two fractions of standard intra-cavitary brachytherapy. Patients with anatomical topography unsuitable for intra-cavitary treatment or those who did not responded well after each insertion of interstitial brachytherapy, received interstitial brachytherapy in all fractions. All brachytherapy insertions were performed by a single radiation oncologist in an outpatient setting under moderate sedation with the use of midazolam and fentanyl, independent of anesthetists. The details of sedation and interstitial brachytherapy technique were previously described. Insertion time (from time out to end of vagina packing) were recorded [6].
Treatment planning was performed with either computed tomography (CT) or MRI, depending on the availability of MRI slots. High-risk clinical target volume (HR-CTV) and intermediate-risk clinical target volume (IR-CTV) was contoured according to European recommendations from Haie-Meder et al. [11], GEC-ESTRO Working Group, and Viswanathan et al. [12], and CT-standardized contour guidelines using an Oncentra Brachy treatment planning software. Organs at risk (OARs), such as the bladder, rectum, and sigmoid colon were also contoured. Brachytherapy plan was optimized to achieve D90 (minimum dose covering 90% of target volume), and HR-CTV was at least 6.5 Gy per fraction while keeping D2cc (minimum dose to the most irradiated 2 cc) to OARs as low as possible. The final planning aim was to achieve HR-CTV D90 of 85 Gy EQD2 or greater; the dose constraint was D2cc of 90 Gy EQD2 or smaller for the bladder, and D2cc of 75 Gy EQD2 or smaller for the rectum and sigmoid. Reduction from four to three fractions of brachytherapy was implemented in cases of good tumor response, where fractional HR-CTV D90 > 8 Gy was achieved while still adhering to dose constraints of OARs. Patients were treated with MicroSelectron HDR (Nucletron), and iridium-192 was used as the treatment source.
Data analysis
Acute complications were assessed during brachytherapy until discharge. Patients were followed up at 1 month post-treatment and then every three to four months for the first two years, followed by every four to six months thereafter for up to five years after completion of radiotherapy. Routine follow-up consisted of evaluation of symptoms, treatment-related toxicities, and physical examination. Imaging was performed if recurrence was suspected. Toxicities were graded according to CTCAE version 5.0. All survival and follow-up details were recorded until July 31, 2021. All statistical analysis were carried out using STATA software version 14.2 (StataCorp LP, Texas, USA).
Results
Thirty-three patients with locally advanced cervical cancers treated with hybrid interstitial and intra-cavitary brachytherapy applicators were included for analysis. The median age was 58 (range, 27-77) years. The majority of patients (n = 31, 93.9%) received concurrent chemotherapy. Eighteen patients (54.5%) underwent a total of three fractions of brachytherapy. Twenty-eight patients (84.8%) were treated with at least 2 fractions of interstitial brachytherapy. Patient and treatment characteristics are summarized in Table 1.
Table 1
Patient and treatment characteristics (n = 33)
A total of sixty-nine fractions of hybrid IC/IS brachytherapy were performed (Table 2). Interstitial ring applicator was used in 10 fractions (14.5%), Utrecht applicator was applied in 32 fractions (46.4%), and Venezia applicator was used in 27 fractions (39.1%). Overall, a total of 320 needles (median of 5 needles per fraction) were implanted, with a median insertion depth of 3 cm (range, 1.5-4.0 cm). The mean doses for midazolam and fentanyl were 2.9 mg per fraction and 59.3 mcg per fraction, respectively. MRI-based planning was carried out in eleven (15.9%) out of sixty-nine fractions, whereas CT-based planning was performed in the remaining fifty-eight (84.1%) fractions. The median insertion time was 28.5 minutes (range, 9-85 min.).
Table 2
Interstitial brachytherapy application characteristics of 69 fractions
The median HR-CTV volume at the time of first brachytherapy within our cohort was 34.5 cc (range, 17.8-74.7 cc). The median fractional D90 HR-CTV was 7.8 Gy (range, 3.6-9.9 Gy), and the median total EQD2 HR-CTV D90 and intermediate-risk clinical target volume (IR-CTV) was 86.1 Gy (range, 76.3-93.1 Gy) and 68.3 Gy (range, 62.2-76.5 Gy), respectively. The median total D2cc (dose to the most irradiated 2 cc of volume) EQD2 of the rectum, bladder, sigmoid colon, and small intestine was 71.8 Gy, 81.5 Gy, 69.0 Gy, and 58.3 Gy, respectively. Larger volume of HR-CTV was predicted for worse local control (HR = 1.08, p = 0.005) and overall survival (HR = 1.04, p = 0.015) (Table 3). A higher HR-CTV D90 dose was shown to be associated with improved overall survival (OS), but not statistically significant (HR = 0.86, p = 0.068).
Table 3
Univariate analysis of local control and overall survival
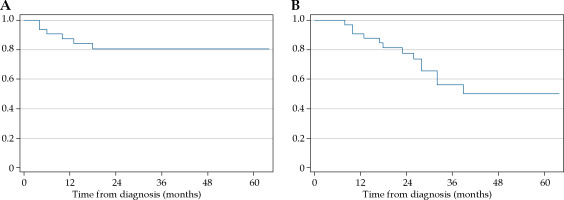
At a median follow-up of 28 (range, 8-64) months, 12 patients died, all from cancer recurrence (distant metastasis in 10 patients and local progression in the remaining 2 patients). In all, 6 patients developed local recurrence and 1 patient had persistent disease post-treatment. Distant metastases were observed in 11 patients. The 2-year local control and overall survival rates were 80.7% (95% CI: 61.2-80.9) and 77.7% (95% CI: 58.6-88.7), respectively (Figure 1A, B). The median HR-CTV volume and total EQD2 HR-CTV D90 in 7 patients with local recurrence and persistent disease was 57.5 cc (range, 33.7-74.7 cc), with 86.1 Gy (range, 76.3-90.8 Gy).
One patient experienced significant bleeding during applicator removal, which required application of Monsel’s paste, in addition to routine vaginal packing with adrenaline. One patient had a mal-placement of uterine tandem within the myometrium. There was no case of the entire uterine perforation or OARs’ perforation. None of the patients required in-patient admission or blood transfusion post-procedure.
Concerning late toxicities, a total of seven patients (21.2%) experienced grade 2-3 gastrointestinal and genitourinary toxicities, four (12.1%) of them had grade 3 toxicities. There were no grade 4-5 toxicities and no procedural-related deaths. One patient suffered from grade 3 cystitis requiring bladder washout and hospital admission. One patient developed recurrent hematuria from grade 3 cystitis that required multiple hospital admissions and a grade 2 proctitis, which was managed conservatively. Two patients experienced grade 3 radiation proctitis that required argon plasma coagulation, and one of them went under hyperbaric oxygen therapy. Two patients had grade 2 proctitis resolved with conservative management. There were no cases of fistulas. One patient underwent cementoplasy for sacral insufficiency fracture. Two patients developed hydrometra from cervical stenosis that was treated with drainage and cervical dilation. The bladder D2cc EQD2 for 2 patients with late grade 3 GU toxicities were 78.1 Gy and 71.1 Gy. The rectal D2cc EQD2 for 2 patients with late grade 3 GI toxicities were 70.1 Gy and 75 Gy.
Discussion
Continuous advances in gynecological interstitial brachytherapy with modern hybrid interstitial brachytherapy applicators and plastic catheters have altered the brachytherapy landscape. This minimally invasive approach substantially reduced procedure-related morbidity while offering excellent treatment outcomes compared with standard intra-cavitary brachytherapy. Data from the RetroEMBRACE study showed that IC/IS brachytherapy increased the 3-year local control rate by 10% in bulky or poorly responding tumors with HR-CTV ≥ 30 cc showing no significant increase in late toxicities compared with intra-cavitary brachytherapy [13]. Murakami et al. recently reported the results of a large multi-institutional retrospective study including a cohort of 469 patients [14]. IC/IS brachytherapy was associated with higher HR-CTV dose and comparable local control rate with IC brachytherapy, despite patients with more advanced stage, bulkier tumor, and poorer tumor respond to external beam radiotherapy.
The present study summarized our experience with hybrid interstitial brachytherapy applicators implanted under moderate sedation with pre-brachytherapy MRI and CT-based planning in the treatment of locally advanced cervix cancer. Our study, with a median follow-up of 28 months in 33 patients, reported a 2-year local recurrence-free survival and overall survival of 80.7% and 77.7%, respectively. About half of the patients in our cohort (54.5%, n = 18) underwent a total of three fractions of brachytherapy, with 12 out of 18 treated with 2 fractions of interstitial brachytherapy and 1 fraction of intra-cavitary brachytherapy. Reduction in the number of fractions from 4 to 3 is feasible for tumors that exhibit a favorable response after first fraction of interstitial fraction, achieving high HR-CTV D90 dose without violating OARs dose constraints. Such modifications to the fractionation schedule based on early response assessment can be implemented to optimize treatment course and potentially reducing overall treatment duration for selected patients. All seven patients with local recurrence had bulky tumor, with HR-CTV volume from 33.7 to 74.7 cc. The predominant site of recurrence was distant. In terms of tolerability, none of the patients in this study experienced a significant complication related to the sedation or applicator and needle placement. In our cohort, only four patients (12.1%) had grade 3 cystitis or proctitis, and none experienced grade 4 toxicity. The results reported in our series are comparable to existing literature on IC/IS brachytherapy (Table 4) [13, 15-22].
Table 4
Studies of image-guided brachytherapy with hybrid intra-cavitary/interstitial applicators
| References | Median follow-up (months) | No. of patients | Type of applicators | Use of pre-brachy imaging | Mode of image- based planning | Mode of anesthesia | HR-CTV volume (median, cc) | HR-CTV D90 | IR-CTV D90 | Bladder D2cc | Rectum D2cc | Sigmoid D2cc | Local control | Overall survival | Incidence of late GI toxicity | Incidence of late GU toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (median, Gy) | ||||||||||||||||
| Present study | 28 | 33 | Interstitial ring Utrecht Venezia | MRI | MRI, 15.9% CT, 84.1% | Moderate sedation | 34.5 | 86.1 | 68.3 | 81.5 | 71.8 | 69.0 | 2-yr, 80.7% | 2-yr, 77.7% | G3, 6.1% | G3, 6.1% |
| Dimopoulus et al. [21] | 20 | 22 | Vienna | No | Yes | Spinal or epidural | 45.6 | 85.0 (mean) | – | 83 | 66 | 67 | 2-yr, 95% | 2-yr, 43% | None G3-4 | |
| Fokdal et al. [13] | 45 | 300 | Hybrid IC/IS | – | MRI, 97% CT, 3% | – | 39.0 | 92.0 | – | 79 | 65 | 65 | 3-yr, 94% 5-yr, 91% | – | G2-5, 14.3% | G2-5, 16.7% |
| Walter et al. [16] | 4 | 10 | Venezia | MRI | MRI & CT | General or epidural | 42.0 | 90.7 | – | 79.4 | 58.7 | 64.8 | – | – | – | – |
| Murakami et al. [22] | 23.3 | 42 | Hybrid IC/IS | MRI | CT | Saddle block or LA & IV sedation | 37.1 | 70.3 | – | 72.4 | 64.5 | – | 2-yr, 80.2% | 2-yr, 81.6% | G3+, 7.2% | |
| Mahantshetty et al. [20] | 35 | 69 | Vienna II | MRI (if available) | MRI, 95.7% CT, 4.3% | General or spinal | 69.0 | 86.0 | – | 86.0 | 68.0 | 68.0 | 3-yr, 76%, 5-yr, 72% | 3-yr, 62% 5-yr, 54% | G3-4, 20% | |
| Keller et al. [15] | 16 | 61 | Vienna Venezia | No | MRI | Moderate sedation | 31.6 | 86.1 | – | 71.8 | 55.4 | 61.2 | 1-yr, 80.6% (LRC) | 1-yr, 86.9% | 1-yr G3+ GI/GU, 5.7% 1-yr G3+ urethral stenosis, 2% | |
| Rivera et al. [19] | 24.9 | 71 | Utrecht | MRI | CT | Moderate sedation | 37.9 | 87.4 | – | 88.3 | 70.0 | 70.4 | 2-yr, 83.6% | 2-yr, 88.6% | G2, 21% G3, 1% | G2, 12% G3, 1% |
| Zhang et al. [17, 18] | 72.3 | 110 | Utrecht Vienna Multichannel vaginal | MRI or CT | MRI or CT | General | 36.7 | 91.3 (mean) | 68.8 (mean) | 77.2 (mean) | 64.7 (mean) | 63.5 (mean) | 3-yr, 90% 5-yr, 90% | 3-yr, 79.1% 5-yr, 76.4% | G2-4, rectum 1-yr, 8.2% 2-yr, 17.3% 3-yr, 18.2% | – |
Other mono-institutional studies have demonstrated similar promising data regarding oncologic outcomes of brachytherapy with hybrid applicators [16, 17, 23, 24]. Rivera et al. published their experience of hybrid tandem and ovoids with Utrecht applicator in 71 patients treated between 2010 and 2017 [19]. In their series, patients had cervical Smit sleeve insertion prior to brachytherapy, and brachytherapy placement was performed in out-patient setting under moderate sedation. With a median follow-up of 24.9 months, 2-year local control (LC) was 83.6%, loco-regional control was 72.0%, and OS was 88.6%. In their cohort, there were two reported cases of treatment-related fistulas (1 recto-vaginal fistula and 1 vesico-vaginal fistula). Another study by Keller et al. reported outcomes of 61 patients treated with Vienna or Venezia applicators [15]. In their cohort, the 1-year overall survival and loco-regional control was 86.9% and 80.6%, respectively. The 1-year incidence of grade 3 and above genitourinary or gastrointestinal late toxicities was 5.7% with fistula occurring in 7 patients. Zhang et al. performed a retrospective analysis on 110 patients with stage 1B2 to IVA cervical cancer [17, 18]. The hybrid applicators were implanted under general anesthesia, and included Utrecht applicator, Vienna applicator, and multichannel vaginal applicator. With a median follow-up of 72.3 months, the 3-year LC and OS were 90.0% and 79.1%, respectively. The 1- and 3-year incidence of grade 2-4 rectum morbidity were 8.2% and 17.3%, respectively.
The importance of MRI in treatment planning for image-guided brachytherapy has been well-established by the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) [11, 25, 26]. Although MRI-based brachytherapy planning in cervical cancer is considered the gold standard, it is however not superior in all aspects. The integration of MRI-based planning into routine HDR brachytherapy has been challenging in many centers in the world, and largely limited by accessibility to MRI scanners, long procedural time, and expensive cost. In our busy tertiary hospital, due to the lack of a dedicated MRI scanner in our department, obtaining an MRI scan at a desired time for every brachytherapy session remains problematic.
In our early experience, we acquire both CT and MR image sets during the first fraction of interstitial brachytherapy until our medical team is confident that target and OARs volume delineation can be performed reliably using CT with pre-brachytherapy MRI. Although we desire to perform MRI planning with applicator in situ, as this is considered the gold standard, it was only possible to arrange an MRI for brachytherapy planning for 11 fractions. In our cohort, all patients had MRI 1 week pre-brachytherapy, and majority (n = 21, 63.6%) had CT-based planning at the time of brachytherapy. Our practice of incorporating MRI in pre-planning setting and using CT only for planning saves procedural time and cost, and is under outpatient practice that can be readily translatable to more radiation therapy centers. Long total procedural time might lead to severe patients’ discomfort, anxiety, and pain [8, 27]. These discomfort had previously been shown to cause movement in patients’ position as well as in the position of needles and applicator, thereby affecting clinical outcomes [28, 29]. Chen et al. reported on applicator placement-related acute side effects during 407 brachytherapy fractions performed in 125 patients [30]. They found that pain and vaginal bleeding were the most common acute side effects, and concluded that reducing total procedural time is helpful to decrease acute side effects, such as hematuria and patients’ discomfort. The results from our previous study showed that the addition of MRI results in an increase of 0.82 hours to the total procedural time compared with CT-based planning [6].
The findings from our study confirm existing evidence on the feasibility and efficacy of hybrid MRI and CT for image-guided brachytherapy. Wakatsuki et al. reported their technique of performing MRI pre-brachytherapy followed by subsequent CT-only based brachytherapy planning [31]. The pre-brachytherapy MRI was applied to pre-plan needle placement for actual insertion, and to facilitate HR-CTV contouring on subsequent CT images at the time of brachytherapy. A similar hybrid MRI/CT technique was reported by Fokdal et al. using MRI for pre-brachytherapy planning with a dummy MUPIT applicator in situ [32]. Murofushi et al. retrospectively reviewed outcomes of 146 patients who underwent pre-brachytherapy MRI within 7 days before first high-dose-rate brachytherapy [33]. They concluded that pre-brachytherapy MRI is beneficial to selected patients who are expected to benefit the most from image-guided adaptive brachytherapy, whilst avoiding the use of inappropriate brachytherapy applicators leading to poor local control.
A systematic review by Wang et al. analyzed 13 studies with a total of 465 patients. The study compared dimensions, DVH parameters of HR-CTV and OARs, and clinical outcomes between CT-only and MRI-based (MRI only or hybrid CT/MRI with at least one fraction of MRI-based brachytherapy) planning in cervical cancer patients [34]. Wang et al. found that in the aspect of HR-CTV dimensions, width was significantly overestimated on CT in all studies, and height could be underestimated when comparing with thickness. As a result, dose parameters for HR-CTV were lower for CT-only approach compared with MRI-based approach. There is only one study that compared clinical outcomes for MRI-only approach with hybrid CT/MRI-based approach, which found comparable cancer control and survival rates. Mahantshetty et al. and a follow-up study by Swamidas et al. have both demonstrated that the CT-based target incorporating real-time trans-rectal ultrasound information was comparable with MRI at the time of brachytherapy [35, 36]. Given the wider availability of CT over MRI, a considerable amount of literature has been published recently by various groups on CT-based contouring consensus to enable more reliable and reproducible target volume delineation [37-39].
In our experience, interstitial brachytherapy using modern hybrid applicators under sedation with pre-brachytherapy MRI to aid CT-based planning, is both time and resource efficient, and can be quickly implemented by radiation oncologists who are experienced in image-based brachytherapy. Despite our encouraging results, some limitations exist in this study, including its single-institution experience, lack of quality-of-life data, and relatively short follow-up. With further maturation of our data, we expect to publish on long-term patient outcomes.
Conclusions
Hybrid IC/IS applicators inserted under moderate sedation is feasible and safe. Image-guided brachytherapy with CT-based planning aided by MRI performed 1 week pre-brachytherapy is associated with favorable outcomes and accepted toxicity profile. This approach present the potential to be cost- and resource-effective.