Dear Editor,
The tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder. Pulmonary lymphangioleiomyomatosis (LAM) occurs in up to 40% of TSC patients and predominantly affects women. Most patients with TSC suffer from epilepsy, and many have cognitive and behavioral problems such as severe intellectual disability, autism, and hyperactivity. We report a case of a young woman who was intubated following convulsive status epilepticus which occurred in a state of clinical stability and who was weaned from orotracheal intubation exclusively using a high-flow nasal cannula (HFNC) with 1.0 FiO2. HFNC provides heated and humidified air with flow rates up to 60 L min−1 with relatively stable oxygen content (adjustable FiO2 0.21–1.0). In this case non-invasive ventilation (NIV) was not used due to the high risk of barotrauma caused by the progression of the cystic lung disease. HFNC proved effective to reduce inspiratory effort, enhance tidal volume by delivering high-flow oxygen and facilitate weaning from ventilators in this patient.
We describe the case of a young, 23-year-old woman known to suffer from TSC-LAM. She was born at full term to healthy nonconsanguineous parents. Her medical history begins at gestational age with the detection of multiple cardiac rhabdomyomas without hemodynamic significance, since when she has been in regular follow-up cardiology for this condition. Furthermore, when she was 14 years old, her clinical history was characterized by bilateral pneumothorax and 3 other episodes of left pneumothorax requiring endopleural drainage. The patient was diagnosed with TSC when molecular analysis revealed she inherited the TSC2 c.1832G > A (p.R611Q) mutation from her mother. The diagnosis of pulmonary lymphangioleiomyomatosis was carried out following the pneumothorax episodes using chest computed tomography (CT) scan showing the presence of numerous cystic airspaces. Abdomen CT scan revealed multiple hepato-renal angiomyolipomas. Also, CT brain scanning revealed diffuse hyperintense areas, which were seen in the bilateral cortex and hypointense cortical tubers with the most voluminous located at the level of the lateral recess of the fourth ventricle and the smallest at the level of the caudate lobe of the right and in the seat of the ipsilateral subependymal ventricle. The electroencephalogram showed electrical brain poorly modulated and unstable activity. She was treated with sirolimus and antihypertensive medication and was not taking any other therapy. Her physical and cognitive development was normal. Following the occurrence of convulsive status epilepticus in a state of clinical stability, loss of consciousness and respiratory arrest, the patient was subjected to tracheal intubation (PS 8 cm H2O, positive end-expiratory pressure [PEEP] 4 cm H2O, Vt 450 mL, FR 12 per min, expiratory cycle 35%, FiO2 0.8) and was admitted to our intensive care unit (ICU). A chest X-ray revealed diffuse bilateral pulmonary cysts and bilateral areas of lung consolidation. Comparing the chest CT scan images acquired in 2014 (Figure 1) with 2020 (Figure 2) shows the evident progression of pulmonary cystic pathology.
FIGURE 1
2014 – computed tomography images. Parenchymal involvement of cystic pathology in the phase of clinical stability

FIGURE 2
2020 – computed tomography images. Parenchymal progression of cystic disease during episode described in this case

Laboratory findings revealed white blood cell count 1.4 G L−1, red blood cell count 3.35 T L−1, hemoglobin 9.0 g dL−1, hematocrit 39.0%, platelets 506 G L−1, aspartate aminotransferase 50 U L−1, alanine aminotransferase 67 U L−1, total bilirubin 0.70 mg dL−1, albumin 2.8 g dL−1, urea nitrogen 50 mg dL−1, creatinine 0.4 mg dL−1, C-reactive protein 5.8 mg dL−1 and procalcitonin 4.5 ng mL−1 on ICU admission. Her vital signs were as follows: heart rate 122 beats per min, blood pressure 150/90 mm Hg, respiratory rate 32 breaths per min, and temperature 38.5°C. Blood culture was positive for Staphylococcus aureus and bronchoaspirate positive for Acinetobacter baumannii. Arterial blood gases on ICU admission showed: pH 7.47, PaCO2 42 mm Hg, PaO2 62 mm Hg, and HCO3− 38.4 mmol L−1 with mechanical ventilation (BiPAP with pressure support 10 cm H2O) and 0.6 FiO2. The patient’s family members refused tracheostomy. Although her oxygenation was not good (PaO2/FiO2 > 106 mm Hg), in order to avoid tracheostomy on ICU day 16 the first weaning attempt was made. The use of NIV was refused due to high risk of barotrauma, but we used a high flow nasal cannula (HFNC) (Fisher & Paykel Healthcare AIRVO 2, Panmure, New Zealand) with 1.0 FiO2. Pulmonary barotrauma can be complicated in mechanical ventilation and refers to the alveolar transalveolar rupture due to the high pressure, causing conditions including pneumothorax, pneumomediastinum, pneumoperitoneum and subcutaneous emphysema. Pulmonary barotrauma may be associated with increased mortality and in some circumstances can be lethal. In general, the barotrauma related to positive pressure ventilation tends to be much less common during non-invasive ventilation, such as nasal high-flow therapy (NHFT), compared to invasive positive pressure ventilation [1]. The patient who was no longer sedated was extubated with support of HFNC with 1.0 FiO2 (Figure 3).
FIGURE 3
Phases of weaning from orotracheal intubation with support of high-flow nasal cannula with FiO2 100%
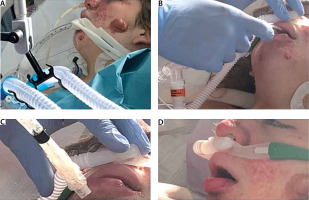
Immediately the respiratory rate decreased from 32 to 22 but showed laryngeal stridor that laryngoscopy confirmed to be due to tubers present on the vocal cords. After 24 hours her vital signs were as follows: heart rate 106 beats per min, blood pressure 140/90 mm Hg, respiratory rate 18 breaths per min, and temperature 38.0°C. Arterial blood gases showed: pH 7.48, PaCO2 41 mm Hg, PaO2 64 mm Hg, and HCO3− 36.4 mmol L−1 with HFNC (flow: 45 L min−1, FiO2 0.8, 37°C). Colimycine and carbapenem antibiotic therapy was started and the patient was transferred to an ordinary hospital 72 hours after extubation. Gradually, in two weeks, the patient weaned from HFNC and was discharged home after independent muscle rehabilitation and with oxygen supplement of 2 L min−1.
The TSC is an autosomal dominant genetic disorder with an estimated incidence at birth of 1 : 6000 newborns [2]. This disorder is caused by inactivating mutations in the TSC1 or TSC2 gene, which are part of the regular operation of the mammalian target of rapamycin (mTOR). The mTOR pathway integrates many mobile inputs to influence a multitude of downstream signaling cascades that are involved in cellular processes such as cell metabolism, growth, proliferation, angiogenesis and survival. It is characterized by the occurrence of various benign tumors in several organs throughout the body. Lesions occur in the brain, kidneys, heart, liver, lungs, and skin. The most common clinical manifestations are abnormalities of the skin (e.g., hypomelanotic macules, facial angiofibromas, shagreen patches, fibrous cephalic plaques, and ungual fibromas), brain (e.g., cortical tubers, subependymal nodules and subependymal giant cell astrocytomas, seizures, and intellectual disability/developmental delay), kidney (e.g., angiomyolipomas, cysts, and renal cell carcinomas), heart (e.g., rhabdomyomas and arrhythmias), and lungs (lymphangioleiomyomatosis); autism and autism spectrum disorders (30–40%); and neurocognitive impairments (50–60%), which often occur in individuals with a normal intelligence quotient [2]. Pulmonary LAM occurs in up to 40% of TSC patients and predominantly affects women. LAM occurs more rarely in patients without TSC (sporadic LAM). It is characterized by cystic destruction of the lung caused by infiltration of smooth muscle cells. Prolonged endotracheal intubation is associated with a number of complications including ventilator associated pneumonia, airway injury, or risk of reintubation. Reintubation is a particular problem especially because of the inability to cooperate with peri-extubation maneuvers, and the relatively high sedation requirements. In adult populations, NIV has been used successfully to treat post-extubation respiratory distress. Mechanical ventilation is a life-saving method [3], which has been proved to improve gas exchange and decrease work of breathing due to fully or partially spontaneous breathing replacement. Unfortunately, invasive mechanical ventilation has been increasingly recognized to be associated with various adverse events, such as ventilator-associated pneumonia and barotrauma. Noninvasive positive-pressure ventilation (NIPPV) may prevent post-extubation respiratory failure and avoid reintubation if it is applied soon after extubation [4, 5]. In addition, according to the most recent guidelines, preventive NIPPV is recommended in patients with high risk of reintubation [6]. However, numerous potential hazards, such as skin damage, eye irritation, interface intolerance, diet or expectoration interruptions might block the use of NIPPV in clinical practice. Recently, high-flow nasal therapy was developed to solve the difficulties of NIV application and is widely used in patients with respiratory difficulty [7, 8]. A conventional nasal cannula or mask cannot evenly deliver O2 to the alveoli when the respiratory pattern is rough and shallow. However, the humidified high-flow nasal cannula is able to deliver a consistent oxygen supply to the alveoli, enabling the patient to maintain a high level of oxygen supply comparable to that obtained using a ventilator. The high-flow nasal cannula is an open respiratory support system. The high flow overcomes labored breathing and generates positive pressure in the alveoli. The difference between the inspiratory volume and the amount of gas delivered to the alveoli is small, and FiO2 is applied relatively consistently. Therefore, high-flow O2 therapy can produce a high positive oxygen concentration in the alveoli by generating positive pressure during spontaneous breathing [9]. Compared with the ‘conventional’ oxygen therapy devices, which deliver gas at 5–20 L min−1 (conventional O2 administration), during HFNC the tracheal inspiratory oxygen fraction (FiO2) is more predictable [10] and the mucociliary function is preserved [11]. In addition, HFNC generates a positive airway pressure (between 2 and 8 cm H2O at the pharyngeal level) which resembles PEEP and is proportional to the administered gas flow rate and varies with the patient breathing pattern (i.e., breathing with the mouth open or closed).
In conclusion, several studies have suggested that prophylactic NIV could reduce the risk of post-extubation respiratory failure in ICU patients at high risk of extubation failure. But in some clinical conditions, NIV is not recommended. HFNC may reduce the inspiratory effort and enhance tidal volume by delivering high-flow oxygen and facilitate weaning from ventilators for these patients. We recommend that the first setting of HFNC flow be the same as the maximum inspiratory flow of the ventilator during pressure support ventilation, and the duration of HFNC be gradually extended in combination with physiotherapy rehabilitation. Further studies are needed to elucidate the efficacy of HFNC during weaning from prolonged mechanical ventilation in a larger population to prevent post-extubation respiratory failure in patients at high risk of extubation failure in the intensive care unit and such a clinical condition where NIV is not recommended.




