COVID-19, caused by the novel severe acute respiratory syndrome coronavirus named SARS-CoV-2, has resulted in thousands of infected critically ill patients admitted to intensive care units (ICUs) worldwide and treated with mechanical ventilation due to acute respiratory distress syndrome (ARDS)-like respiratory failure, since the end of 2019 [1]. In a case series of 1591 patients admitted to hospitals in Lombardy (Italy), 88% required invasive mechanical ventilation. The median length of stay in the ICU of all discharged patients was nine days. Nevertheless, 58% of the patients were still in the ICU at the time of writing of the Italian paper [2]. This implies that these patients were hospitalised in the ICU for one to more than five weeks.
Percutaneous tracheostomy (PT) in the ICU is widely used to facilitate weaning from mechanical ventilation, to anticipate prolonged mechanical ventilation, to aid in the management of respiratory secretions and protect the airway in patients at risk of aspiration, to prevent laryngeal injury, and to minimise sedatives [3, 4]. PT is a surgical procedure to create airway access through the anterior wall of the trachea. It is one of the most common surgical procedures performed in the ICU [5]. In surgical tracheostomy, dissection of the pretracheal tissues and incision of the anterior tracheal wall are followed by inserting a tracheal cannula under direct vision. In percutaneous tracheostomy, blunt dissection of the pretracheal tissues and dilatation of the anterior tracheal wall using the Seldinger technique are followed by insertion of a tracheal cannula, with or without bronchoscopic guidance. Although there are many different PT techniques, in our centre we largely perform the guidewire dilating forceps tracheostomy. This technique was invented by the Australian surgeon Griggs in 1990. In this technique, a specially designed forceps (modified Howard Kelly forceps) is placed over a guidewire after puncturing the trachea and is used to dilate the pretracheal tissues and anterior tracheal wall, followed by placing the tracheal cannula over the guidewire and inserting it into the tracheal lumen [6].
Perioperative complications of percutaneous tracheostomy consist mainly of bleeding, puncturing the posterior tracheal wall or the cuff of the endotracheal tube, tracheal fracture, cannula misplacement, and conversion to an alternate technique. Among the early complications are bleeding requiring further management, the formation of granulation tissue around the tracheostomy, infection, cuff leakage, accidental decannulation, and others. Late complications consist mainly of tracheal stenosis, stridor, tracheoesophageal or tracheocutaneous fistula, cosmetic problems, and others [5, 7].
Clinical practice has been altered profoundly by the COVID-19 setting, with safety for healthcare practitioners being of cardinal importance. New strategies and protocols needed to be set up for safe conduct of procedures such as tracheostomy as well as general management of ICU [8]. Reallocation and provider loss because of COVID-19 illness in physicians and other healthcare providers has challenged the functioning of the medical taskforce. With regard to tracheostomy in COVID-19 ICU patients, recommendations vary widely worldwide. These include recommendations from the American Academy of Otolaryngology – Head and Neck Surgery recommending “Tracheotomy can be considered in patients with stable pulmonary status but should not take place sooner than 2 to 3 weeks from intubation and, preferably, with negative COVID-19 testing” [9]. More restrictive recommendations have been suggested by the American Association for the Surgery of Trauma, who state “At this time, we recommend against performing tracheostomy in patients with active COVID-19 disease” [10]. Conversely, we believe that adopting a safe and reproducible tracheostomy technique may improve the clinical outcome of critically ill COVID-19 patients requiring prolonged intubation.
In this article we propose a standardised approach for tracheostomy, including patient selection, additional COVID-19-specific precautions, detailed checklists as well as preliminary results of our own experience. Our findings are also useful to guide safe performance of tracheostomy in COVID-19 patients to facilitate ventilator weaning.
METHODS
Aim and design
A new practical approach and protocol for PT in COVID-19 patients was set up in the ICU department of the Universitair Ziekenhuis Brussel, alongside with anaesthetic and bronchoscopy considerations and people management of percutaneous tracheostomy in a COVID-19 setting. We performed a retrospective analysis with clinical data available from the medical files of ICU patients with a PT. Our data were collected between 25 March 2020 and 15 May 2020. The aim of our study was to see if a percutaneous tracheostomy could be safely performed, for both patients and healthcare providers, in COVID-19 patients, in order to facilitate ventilator weaning. Complications were categorised as minor or major in parallel with other studies [7, 11]. The Institutional Review Board and Ethics Committee of the Universitair Ziekenhuis Brussel agreed with the retrospective observational nature of the study and waived informed consent (BUN2020-216).
Patient population
Our patient population consisted of critically ill patients with COVID-19 admitted to the ICU Department of the Universitair Ziekenhuis Brussel, treated with mechanical ventilation and percutaneous tracheostomy. The first 16 patients treated with percutaneous tracheostomy were included in our study. Clinical data on the follow-up of patients with a tracheostomy were collected and analysed for quality control reasons to optimise practice.
Statistical plan
All data were anonymised. Demographic data are expressed as median (interquartile range, IQR). Perioperative complications and early complications are expressed as total number (percentage). The statistical analysis was performed using Excel (Microsoft, Redmond, Washington, USA).
Practical clinical approach
Procedure
To achieve an optimal yield of the procedure (benefit of PT versus risk for staff and use of materials) planning was of cardinal importance: patient selection (survival scoring systems, multi-disciplinary consultation, viral status), the timing of the procedure, and consent of family members were essential.
To limit the exposure time to high COVID-19 viral load during PT, a strict working order was developed (Figure 1A). A bedside protocol was designed covering topics of risk analysis (clotting status, haemoglobin level, anatomy and relevant medical history, enteral feeding status, current medical therapy including low-molecular-weight heparin, etc.), device issues (protective goggles, face shields, FFP3 [filtering facepiece class 3] masks, tracheostomy sets, sterile material, VIDEO bronchoscopy), drug management (analgesia, hypnotics, and muscle relaxants), people management (surgeon/operator, anaesthesiologist, pneumologist, back-up surgical team, nurses), and a procedural surgical checklist. A single page checklist was present in every ICU and was run through before the actual launch of the procedure (Table 1).
FIGURE 1
Percutaneous dilational tracheostomy in COVID-19 setting. A) Four ICU team members performing the procedure (anaesthesiologist, bronchoscopist, two surgeons). B) Sterile table setting: required material for percutaneous tracheostomy. C) Three members of the Corona Cannula Taskforce
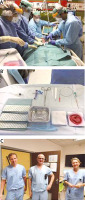
TABLE 1
Single page checklist for percutaneous tracheostomy in COVID-19 patients
Because of the presence of nurses who were unfamiliar with the procedure, a laminated picture of the necessary material was present in every ICU unit (Figure 1B). Informative material was made available to all healthcare providers (HCPs) involved, to facilitate optimal preparation.
Medical workforce: foundation of the Corona Cannula Taskforce
In the second week of April 2020, more than 35 critically ill COVID-19 patients were simultaneously treated for respiratory failure in the central and new remote ICUs (Coronary Care Unit, Recovery Room, and Converted Haemodialysis Ward). According to the phased approach, ICU staff reached out to “back office” colleagues: surgeon and ENT involvement was ready on April 9th 2020. When the decision was made to perform PT, the COVID-19 Control Room contacted the coordinator of the Corona Cannula Taskforce. This Taskforce consisted of seven surgeons (thoracic, endocrine, and abdominal surgery departments) with experience with PT (Figure 1C). They performed the procedure in combination with anaesthesiologists and pneumologists from the ICU department. All non-ICU operators were introduced to the COVID-19 setting and performed one procedure with the surgeon-intensivist of the ICU Department to secure optimal preparation and minimise errors against procedures and safety.
To optimise follow-up and troubleshooting, the ENT engaged themselves to design a PT-guideline. This included safety measures such as guidance on aspiration techniques and a safe approach to inner cannula replacements (Table 2). A unique digital enhanced cordless telecommunications (DECT) phone number was communicated. Clinical pro-active follow-up of the ICU PT patients was performed by one of the surgical operators.
TABLE 2
Otorhinolaryngology percutaneous tracheostomy guideline
Anaesthetic considerations
Critically ill COVID-19 patients are still assumed to be infectious at the optimal window of PT placement, so all participants use maximal protection measures including medical gowns, protective face shields, and FFP3 masks conforming to local protocol [13]. Also, the number of caregivers is restricted because of the high risk of virus-containing aerosol production, so preparation and anticipation are more than ever key to a successful placement and, if necessary, crisis management. Minimal equipment consists of analgesia, a hypnotic of choice, and high-dose muscle relaxant to reduce the risk of coughing and aerosol formation [14, 15]. Furthermore, resuscitation fluids and vasoactive agents, including epinephrine and atropine, should be within arms’ reach of the anaesthesiologist. Lastly, a difficult intubation cart (including a video laryngoscope and spare endotracheal tubes, a self-inflating breathing bag, etc.), oxygen, and aspiration are necessary in case the surgical procedure fails and there is an indication for urgent reintubation. The patient’s file is reviewed to look for reports of previous difficult intubation.
After the patient is induced and paralysed, optimal positioning is achieved in consultation with the surgeon. The patient is ventilated with the fraction of inspired oxygen (FiO2) set at 100% five minutes before the procedure. Before the introduction of the bronchoscope, the ventilation is halted temporarily, and the endotracheal tube (ETT) is clamped [12], to reduce aerosol formation. The ETT is partially withdrawn after another cessation of ventilation and cuff deflation. Tube position during withdrawal is checked by bronchoscopy. After puncture of the trachea, ventilation is again paused for tracheal dilatation and only restarted after placement of the tracheal cannula, inflation of the tracheal cuff, and proper connection to the ventilator. A successful placement is confirmed using bronchoscopy.
Bronchoscopy considerations
Bronchoscopy is a widely used technique to assist in percutaneous tracheostomy [16], although no randomised controlled trials have investigated its effect [17]. Our current protocol also recommends this technique because it reassures the physician performing the procedure of a correct cannula placement by giving additional visual assistance during several steps of the procedure: while inserting the needle, to confirm the progression of the guidewire distally in the direction of the lungs, to confirm dilatation of the trachea, and finally to confirm proper placement of the cannula inside the trachea. Moreover, the bronchoscopy allows removal of any blood from the airway that might have oozed into the trachea during the procedure. Additional precautions were taken during the COVID-19 pandemic. To prevent cross-contamination of patients, disposable bronchoscopes were used. As mentioned before, to minimise aerosolisation of the virus into the air when opening the ventilation circuit, the tube is clamped and the ventilation is stopped. This is done when a swivel joint with valve and opening surrounded by a soft sealing is placed or removed and when the bronchoscope is inserted or removed. During the first procedures, the swivel joint and the bronchoscope were inserted in different steps; however, as experience grew, it was performed at the same time. While the bronchoscopist awaits further steps of the procedure, the bronchoscope is retracted until it is positioned only a few millimetres inside the ventilation circuit, in order to avoid compromising ventilation and to avoid risks of aerosolisation if it is totally removed. When the cannula is in position, the bronchoscopy is inserted through the newly placed cannula, which allows the operator to check the position and whether there is any residual endoluminal bleeding.
RESULTS
First clinical experience
Demographics of patients in whom a guidewire dilating forceps tracheostomy was performed are shown in Table 3. The median age of our patients was 62 years. Our population consisted of 69% males, with a median total body mass index (BMI) of 30 kg m-2. The median number of days of mechanical ventilation until tracheostomy was 18, and the median number of days until decannulation was 20. The median APACHE II (acute physiology and chronic health evaluation) score for our population was 20, which corresponds to a predicted mortality of 36%. The median SAPS II (simplified acute physiology score) score was 36, corresponding to a predicted mortality of 18%, and the median SOFA (sequential organ failure assessment) score was 8, which corresponds to a predicted mortality of 15–20%.
TABLE 3
Patient demographics
Perioperative complications of percutaneous tracheostomy are shown in Table 4. Perioperative complications were defined as related to the procedure and occurring during the procedure or within the first 24 hours after the procedure. The overall perioperative complication rate was 3 out of 16 patients, or 19%, but with two minor superficial skin bleedings treated with compressive skin suture for three days, and one significant skin wound requiring a skin suture. There were no major perioperative complications in our study.
TABLE 4
Perioperative complications
Early complications, defined as related to the procedure and occurring between the first 24 hours and the moment of decannulation, are shown in Table 5. The overall early complication rate was 3 out of 16 patients (19%): two minor superficial bleedings that required a compressive skin suture and one skin infection (erysipelas at the neck level) that was treated with antibiotic therapy for one week. There were no major early complications due to the procedure. Four patients died due to multiple organ failure before ever being decannulated.
TABLE 5
Early complications
None of the healthcare providers involved in performing percutaneous tracheostomy in COVID-19 patients in the ICU developed any clinical symptoms, nor were any diagnosed with COVID-19.
DISCUSSION
The COVID-19 pandemic has resulted in a massive number of long-term ventilated ICU patients [1]. To provide optimal care combined with the safety of healthcare providers, the entire concept and strategy of percutaneous tracheostomy was revised. Very recently, the first publications appeared on the adapted procedures and protocols in a COVID-19 setting [18, 19]. This is of great value to the medical community because we face the same challenges in this unforeseen and rapidly evolving worldwide pandemic event.
The practical clinical approach described herein includes both logistic issues and personnel management. As reported by De Waele et al., a quick, transparent, and workload-based structure proved to be of primary importance in the level of readiness of ICUs around the world [8].
From the demographic data of our patients, age, BMI, and higher percentage male population correspond with being risk factors for COVID-19 viral infection. Intubation time until tracheostomy is longer in comparison to other studies in non-COVID-19 patients [7, 11]. In general, long-term ventilation in ICU means mechanical ventilation for more than 10 to 14 days. A meta-analysis by Huang et al. involving five studies and 406 participants, showed no consistency about the specific timing of performing a tracheostomy in critically ill patients. Early tracheostomy had no significant effect on short-term or long-term mortality and was not associated with a distinctly reduced duration of invasive mechanical ventilation or length of stay in the ICU [20], although, a systematic review and meta-analysis by Griffiths et al. did indeed show a marked reduction in these last two parameters [21]. The LUNG SAFE study, with the inclusion of 459 ICUs across 50 countries, showed a median timing to tracheostomy of 14 days after the onset of ARDS [22]. The median cannulation time was 20 days, but two patients were still cannulated at the time of writing this article, and four patients died due to multiple organ failure. This corresponds to a mortality rate of 25% (4 out of 16 patients), while the predicted mortality rate calculated with the median APACHE II score was 36%, the median SAPS II score was 18%, and the median SOFA score was 15–20%. These different ICU mortality models were measured after the first 24 hours of admission, while some patients were only intubated on their third day of admission to the ICU.
Other studies show variable perioperative complication rates with this technique, ranging from 19% [23] through 25% [7] to 52% [11], but in all these studies percutaneous tracheostomy was performed in patients before the COVID-19 pandemic. For example, one of our minor complications was a significant skin wound, requiring a skin suture. This was probably due to the lower visibility during the procedure as a result of fogging of the protective face shield. The overall complication rate of 15% [7] and 22% [11] in patients while cannulated, which is found in other studies, is in line with our numbers, although in our study we did not experience any major complications. One study of tracheostomy in COVID-19 patients, by Angel et al., not yet published at the time of writing this article but seen as a journal pre-proof, showed no intra-procedural complications and only minimal post-procedural complications (5.1%), but with one patient needing a surgical exploration and two accidental decannulations [18]. In our study, these last three complications are seen as major complications. Our patient group was rather small, being only preliminary results of our own experience; also, four patients died due to multiple organ failure. Most of our complications were superficial bleeding, this was probably due to the fact that all COVID-19 ICU patients were under high-prophylactic doses because of the higher risk of thrombotic complications [24].
To our knowledge, there is only one other study reporting on perioperative complications or early complications after percutaneous tracheostomy in COVID-19 patients. This study was not yet published at the time of writing this article. Therefore, it is difficult to compare our results with other studies, performed before the COVID-19 pandemic. The COVID-19 pandemic compelled to many safety precautions to protect healthcare workers from infection. Circumstances for performing the procedure were difficult (lower visibility with protective glasses and covering face shields, higher temperature at the ICU because room ventilation is prohibited to avoid spreading of the virus, etc.). In our study, we only used the guidewire dilating forceps tracheostomy technique, because this is the procedure with which we have the most experience in at our centre and we decided not to introduce a new unfamiliar technique in this challenging setting.
CONCLUSIONS
Percutaneous tracheostomy is a key component of the care of critically ill ventilated victims of the COVID-19 pandemic in the ICU. Procedures were adapted to ensure maximised safety for both patients and healthcare practitioners involved in the procedure. This COVID-19-centred strategy based on flexibility, preparation, and cooperation between healthcare providers with different backgrounds facilitated percutaneous tracheostomy in COVID-19 patients, and our initial data reveal no overt complications at this point in time for patients nor for healthcare practitioners. Thus, our findings provide initial evidence that tracheostomy can be performed safely as a standard of care for COVID-19 patients requiring prolonged mechanical ventilation as has been standard practice in ICU patients prior to the COVID-19 pandemic to promote ventilator weaning and patient recovery.




