Postoperative infection remains one of the major complications that hamper perioperative management. Such infections after cardiac surgery often cause serious and life-threatening injury to patients [1, 2]; therefore early diagnosis and prompt treatment are crucial [3]. Though biomarkers such as procalcitonin, interleukin-6, C-reactive protein (CRP) levels, and white blood cell (WBC) count are routinely used for early diagnosis of infection, prompt diagnosis of postoperative infection remains difficult because inflammatory markers also increase in the early perioperative period due to surgical stress [4]. Thus, a novel biomarker that is specific for the early diagnosis of infections is required.
Presepsin is a biomarker of an inflammatory response. It is a 13 kDa protein, a soluble subtype of cluster of differentiation (CD) 14 protein, the receptor for lipopolysaccharide-binding protein complexes, which is expressed in granulocytes, monocytes, and macrophages. Presepsin is generated by inflammatory plasma-protease activity and is released into the circulation after activation by lipopolysaccharides following the detection of infectious agents. Therefore, presepsin levels increase due to bacterial phagocytosis of monocytes or macrophages [5–7]. Moreover, the presepsin response should be more rapid than that of the other standard inflammatory markers. Its response time has been reported to be only 2–3 hours in certain animal studies [8]. Many studies have reported that presepsin is useful not only for the diagnosis of infectious diseases, but also for predicting the severity, prognosis, and effectiveness of treatment [7, 9–11]. Therefore, in perioperative management, presepsin could represent a more useful marker for early detection of postoperative infection than the currently available markers. However, there are limited reports on the utility of presepsin in the perioperative period. Thus, this study aimed to investigate the relationship of preoperative or early postoperative presepsin levels, and the incidence of postoperative infection after cardiac surgery.
METHODS
This single-centre, prospective, observational study was approved by the Ethics Committee of the Yamagata University Faculty of Medicine. All patients provided written informed consent prior to surgery. We enrolled patients over 18 years of age who were scheduled to undergo elective cardiac surgery with cardiopulmonary bypass (CPB) between August 2015 and December 2017 at the Yamagata University Hospital, Yamagata, Japan. Exclusion criteria were emergency surgery, renal failure, preoperative infectious disease within 30 days, and reoperation within 30 days after surgery (except for procedures related to infectious disease such as abscess drainage).
The induction and maintenance of anaesthesia was performed with midazolam, propofol, sevoflurane, and fentanyl with remifentanil. Neuromuscular blockade was performed with rocuronium bromide. We inserted arterial, central venous, and pulmonary catheters in all cases for perioperative management. Intravenous cefazolin (1 g) was administered prophylactically prior to starting surgery. An additional intraoperative dose of 1 γ was administered every 3 hours until the end of surgery. In addition, all patients received 500 mg of intravenous methylprednisolone after the induction of anaesthesia and before commencing surgery. All patients were admitted to the intensive care unit (ICU) of our hospital after surgery.
Surgical techniques, criteria for extubation, discharge from the ICU, admission to a high care unit (HCU), and other perioperative management measures for all patients included in this study were performed according to the standard procedures of our hospital. Surgeons and physicians who were responsible for postoperative management were blinded to the data regarding presepsin levels in this study.
Concentrations of presepsin were determined by PATHFAST Presepsin assay (LSI Medience Corporation, Tokyo, Japan). The presepsin levels were measured before surgery (after induction of anaesthesia), after completion of the surgical procedure as postoperative day (POD) 0, and additionally in the morning (about 7–9 A.M.) of the day after surgery as POD 1 in all patients enrolled from May 2016 until the end of the study. Other inflammatory cytokines were measured every day during ICU admission, and at the surgeon’s discretion after discharge from the ICU.
The primary outcome in this study was the relationship of perioperative presepsin levels with the incidence of postoperative infectious complications within 30 days after surgery. Perioperative data, including postoperative infectious complications, were collected by a retrospective review of medical records. The criteria for diagnosing infectious complications were all included as follows: infectious complications occurring after POD 4; presence of signs of infection (fever > 38.0°C, re-elevated or persistent WBC count ≥ 10 G L-1, local pain, tenderness, swelling, heat, or purulent discharge); and the presence of positive cultures or any interventions performed for the treatment of infections (e.g., anti-biotics or drainage), based on the clinical decision of the physician.
Additionally, we collected the following data of factors that may be associated with postoperative infection: preoperative demographic data; American Society of Anesthesiologists-Physical Status (ASA-PS) classification; the European System for Cardiac Operative Risk Evaluation (EuroSCORE) that identifies a number of risk factors which help to predict mortality due to cardiac surgery [12]; pre-existing diabetes mellitus; preoperative blood sample analysis including WBC count, C-reactive protein (CRP) and procalcitonin (PCT) levels; duration of anaesthesia, surgery, CPB, intubation, postoperative ICU stay, and hospital stay; and blood examination on POD 0.
The sample size calculation was based on our previous preliminary study. A standard deviation of 420 pg mL-1 and a difference in mean presepsin of 440 pg mL-1 on POD 0 were observed when comparing 7 infected with 21 non-infected patients in the study. To show that differences were statistically significant, it was estimated that at least 20 patients per group were needed using a type 1 error rate, 2-sided, of 5% and a power level > 90%. Differences with a p-value < 0.05 were considered statistically significant. Categorical data were presented as frequency (%). Continuous data were presented as median [interquartile range] or mean standard deviation. Variables were compared between the infected and non-infected group using the unpaired t-test, paired t-test, or Wilcoxon’s signed-rank test. Risk factors for postoperative infectious complications were examined using univariate logistic regression analysis. In addition, we performed multivariate logistic regression analysis to evaluate the effect of potential confounding independent variables. Receiver operating characteristic (ROC) curve analysis was conducted to define the optimal cut-off values. Kaplan-Meier analysis and the log-rank test were used to compare infection-free survival between the groups, divided by the cut-off value of presepsin. Statistical analysis was performed using R software (version 3.4.1, R Foundation for Statistical Computing, Vienna, Austria) and EZR (Easy R) version 1.36 [13].
RESULTS
Between August 2015 and December 2017, 80 patients were recruited to our study. Of these, 7 were excluded due to re-operation (bleeding, etc.) within 30 days after surgery. Thus, 73 patients were enrolled for analysis. Forty-three patients who were recruited from the latter period of May 2016 to the end of the study underwent presepsin measurement on POD 1. The details of the procedures were single valve surgery (n = 46), aortic surgery (n = 6), patch closure for congenital disease (n = 3), tumour resection (n = 1), and combined surgery (n = 17). The characteristics of the patients and details of surgery are shown in Table 1.
TABLE 1
Patient characteristics (N = 73)
Of the enrolled patients, 20 (27%) developed po-stoperative infectious complications within 30 days after surgery. The mean time to onset of infectious complications after surgery was 9.5 (4–29) days. The infections included mediastinitis (n = 5), superficial wound infection (n = 4), bloodstream infection (n = 2), urinary tract infection (n = 5), pneumonia (n = 2), and unidentified infectious focus (n = 2).
The mean serum presepsin level on POD 0 was significantly higher in the infected group than the non-infected group. Moreover, the mean preoperative presepsin level was significantly higher in the infected group than the non-infected group. The presepsin levels on POD 1 were not significantly different between the groups. In contrast, there were no significant differences in the WBC, CRP, or PCT levels before surgery and on POD 0 between the infected and non-infected groups (Table 2). The durations of surgery, CPB, postoperative hospital stay, ICU/HCU stay, and ventilation were significantly longer in the infected than the non-infected group. In the Tukey method with ANOVA, we found only one indication where the presepsin level on POD 0 after combined surgery was significantly higher than that after single valve surgery (568 ± 379 pg mL-1 vs. 313 ± 173 pg mL-1, P = 0.036). However, the difference was lost with Bonferroni or Holm correction. Additionally, with Pearson’s correlation analysis, there was a positive correlation between the presepsin value on POD 0 and surgery duration (r = 0.388, P < 0.05).
TABLE 2
Comparison of characteristics of non-infected and infected patients
[i] Categorical data are presented as number (%), continuous data are presented as mean ± standard deviation or median [interquartile range].
[ii] DM – diabetes mellitus, CPB – cardiopulmonary bypass, RCC – red cell concentrates, ICU – intensive care unit, HCU – high care unit, WBC – white blood cell count, CRP – C-reactive protein, PSEP – presepsin, PCT – procalcitonin, POD – post-operative day, ΔPSEP – PSEP level on POD 0 minus pre-operative level
The risk factors determined using the univariate and multivariate analyses are shown in Table 3. The risk factors for postoperative infectious complications were presepsin levels at the preoperative baseline and on POD zero. In multivariate logistic regression analysis, only the preoperative baseline presepsin level was identified as an independent risk factor of postoperative infectious complications.
TABLE 3
Risk factor analysis of postoperative infection
According to ROC curve analysis, the cut-off values of preoperative and POD 0 presepsin level were 132 pg mL-1 (sensitivity; 0.650, specificity; 0.885) and 347 pg mL-1 (sensitivity 0.750, specificity 0.642), respectively (Figure 1). Kaplan-Meier analysis was performed to examine the accuracy of these cut-off values (Figure 2). Significant differences were observed in infection-free survival rates between the groups when divided by the cut-off values of presepsin for the log-rank test.
FIGURE 1
Receiver operating characteristics curves of presepsin concentrations (A) on POD 0 and (B) preoperatively with postoperative infection. Areas under the curve were (A) 0.674 (0.537–0.811) and (B) 0.817 (0.704–0.930)
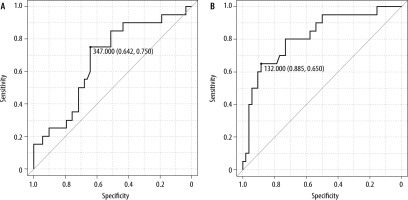
FIGURE 2
According to Kaplan-Meier analysis and the log-rank test, there were significant differences in terms of infection-free survival between the groups divided by presepsin cut-off values (A) on postoperative day 0 and (B) preoperatively. There were significant differences (P < 0.05) between groups in the log-rank test. PSEP – presepsin

DISCUSSION
The results of this study provide suggestive evidence that serum presepsin levels in the early perioperative period could be used as an early marker to predict postoperative infectious complications after cardiac surgery. Furthermore, we demonstrated that the preoperative presepsin level is an independent predictor of postoperative infectious complications after cardiac surgery with CPB.
In this study, presepsin and WBC levels were elevated on POD 0, despite the elevation of CRP and PCT levels only after POD 1. This might be due to the difference in the release (or response) time. The release time of presepsin is approximately 2 hours [8], which is faster than that of CRP or PCT. Conversely, WBC levels were elevated on POD 0 similar to presepsin levels in our study. Generally, WBC levels are the quickest to rise among the inflammatory markers used in routine clinical practice. The rapid elevation of WBC levels is not due to increased production but due to migration from the bone marrow to the blood stream. In addition, increased neutrophil activity during CPB might increase WBC during the early postoperative period. However, there was no significant relationship between WBC levels on POD 0 and postoperative infection, despite the elevated WBC levels in both groups in our study.
We propose two possible hypotheses regarding the relationship between early perioperative presepsin and the risk of postoperative infectious complications. Firstly, preoperative subclinical infections or infectious agents might be reflected by the preoperative presepsin value. Preoperative bacterial carriage could affect the incidence of postoperative infection, as observed in a previous report which showed that preoperative bacterial carriage increased the risk of surgical-site infection in major heart surgery [14]. Moreover, the intraoperative increase in presepsin levels might indicate bacterial invasion of a surgical wound, tubes, or catheters inserted for perioperative management.
Secondly, we hypothesize that other causes of inflammation might affect perioperative presepsin values and postoperative infection. A recent report showed perioperative presepsin values to be associated with cardiovascular and cerebrovascular complications and mortality, regardless of infection [15, 16]. Handke et al. concluded that the presepsin level could reflect cardiovascular sclerosis due to vascular microinflammation. In addition, a few studies have reported that other soluble CD14 subtypes are associated with cardiovascular disease [17–19]. Therefore, it is possible that the preoperative presepsin level indicates the degree of cardiovascular disease, although it has been suggested that presepsin levels only increase due to bacterial infection.
Bomberg et al. [16] reported that preoperative presepsin was an independent predictor of short and long-term mortality after elective cardiac surgery. An association of preoperative presepsin with long-term mortality could suggest that preoperative presepsin level might reflect the severity of cardiovascular disease of the patients. In contrast, two-thirds (18 [67%] of 27) of the non-survivors died because of ‘systemic inflammatory response syndrome or sepsis’ within 30 days after cardiac surgery. Their results imply that the infectious complications had a significant impact on the 30-day mortality of the patients with higher presepsin levels. The results of this study might support the fact that any infectious complication might be associated with the 30-day mortality of the patients with higher preoperative presepsin levels after cardiac surgery.
Furthermore, surgical stress such as the procedure or duration of cardiac surgery might also affect early postoperative presepsin values. Our results indicated that there might be a relationship between postoperative presepsin values and intraoperative surgical stress from the procedure or duration of cardiac surgery. Preoperative inflammation could be enhanced by secondary surgical stress, which might subsequently induce a greater compensatory anti-inflammatory response syndrome (CARS) [20–22]. Induction of CARS may cause immunoparalysis and remote onset of infection after surgery [23].
Despite the above-mentioned reasons, the present study shows that preoperative presepsin levels might indicate pre-existing inflammation which is undetectable by existing conventional inflammatory biomarkers such as WBC count or CRP/PCT levels.
Popov et al. [24] reported that presepsin value on POD 1 was associated with postoperative infection in cardiac surgery. This study revealed additional evidence that the measurement of presepsin levels preoperatively and on POD 0 could predict the risk of postoperative infection, which might be important because early detection and intervention are crucial for successful treatment. However, there seems to be some discrepancy with their result. In this study, the presepsin values on POD 1 had no significant association with postoperative infection. In contrast, Popov et al. reported that preoperative presepsin levels had no significant association with postoperative infection, and values on POD 1 were higher among patients who later experienced infectious complications within 30 days after cardiac surgery than in an equivalent patient population with no infectious complications. We believe that these discrepancies in the results were due to the following: in the study of Popov et al., the preoperative values of presepsin in the infected and non-infected groups (140 and 131 pg mL-1) seemed to be higher than those of the non-infected group (93.1 pg mL-1) of our study. Moreover, the preoperative median CRP value of their study population could be higher than that of our study. Furthermore, the incidence of infection (37%) in Popov’s study was higher than that of our study (27%). Therefore, there could be some differences in preoperative conditions or the perioperative management between Popov’s study and this study. Moreover, there might be a difference in the criteria of postoperative infection. The criteria of postoperative infection were not clarified in Popov’s study.
Presepsin was measured on POD 1 for the latter part of the patients in the study group. Presepsin was not measured in 30 patients on POD 1 because of a change in the study design during the research period. We added POD 1 to the points of measurement of presepsin from May 2016 to the end of the study. Therefore, the participants included in this study after May 2016 (n = 43) all had presepsin values measured on POD 1. Consequently, the evaluation of the presepsin values on POD 1 in this study might be unreliable because of the small sample size. However, we particularly focused on the preoperative and POD 0 presepsin values as the ‘early’ predictor for postoperative infection during cardiac surgery. We were certain that the values of presepsin preoperatively and on POD 0 were more useful predictors of postoperative infection than those on POD 1 in clinical practice, though the evaluation of POD 1 in this study might be unreliable.
We found some associations of the preoperative and early postoperative presepsin values with the entire postoperative infection. However, the various infection sites (such as the surgical site, urinary system, respiratory system, etc.) could not be differentiated according to the presepsin values because of the small sample size of this study. Further studies with larger populations are warranted.
We excluded patients with renal dysfunction because such patients could have increased presepsin levels in the absence of infectious conditions [12].Therefore, serum presepsin levels might not be helpful for early diagnosis of infection in patients with renal failure.
There were some limitations to this study. Firstly, the basic mechanism of perioperative increase in presepsin levels is still unclear. Secondly, we did not evaluate other cytokine levels or biological assessments during the early perioperative period that could support our hypothesis. Further research may be needed to verify the aforementioned correlations. Third, the results of our study might not be generalizable, as this was a single-centre study, although the infection rate in our study population was similar to that reported in a European multicentre survey [25].
Although many factors are left to be explored, our results suggest that identifying elevated risk of infection in the early perioperative period could decrease the incidence of infection and improve outcomes of patients undergoing cardiac surgery through early preventive intervention. Routine examination of presepsin levels during the early perioperative period in cardiac surgery could have clinical benefits. In this study, some patients displayed re-elevated high levels of WBC and CRP during the late postoperative period after cardiac surgery. Some of them subsequently developed postoperative infection. Early intervention with reference to preoperative or early postoperative presepsin values might prevent development of postoperative infection and improve outcomes of the patients. Our results are evidence that the early perioperative presepsin value could be helpful to predict postoperative infection, early intervention, and improvement of the prognosis of these patients. Furthermore, it might be effective to administer preoperative prophylactic antibiotic for patients with higher presepsin values, or switch to broader-spectrum antibiotics or increase the dosage. Moreover, preoperative presepsin values might provide a numerical target for assessment of preoperative interventions to prevent postoperative infection in cardiac surgery.
CONCLUSIONS
Our results suggest that preoperative and early postoperative presepsin levels could be used as markers to predict postoperative infection after cardiac surgery. This method could be useful in identifying patients at high risk of postoperative infection, which could subsequently facilitate treatment at an early stage, resulting in improved outcomes after cardiac surgery. We are currently conducting further research to demonstrate the utility of presepsin as an early predictor of infection after cardiac and other high-risk surgical procedures.




