Dear Editor,
We would like to share the case of a woman whose unrecognized pheochromocytoma presented suddenly as severe heart failure and cardiogenic shock with an “inverted” takotsubo cardiomyopathy progressing to cardiac arrest, requiring hospitalisation in an intensive care unit and emergency adrenalectomy.
Pheochromocytoma is a rare catecholamine-secreting neuroendocrine neoplasm arising from the chromaffin cells of the adrenal medulla. In 10% of cases it is extras-adrenal, it can be associated with family syndromes (Multiple Endocrine Neoplasia-type 2, Von Hippel-Lindau, Sturge-Weber), and in 90% of cases it is a benign neoplasm – more rarely it can also be bila-teral. Richly vascularized, it can present with necrotic or haemorrhagic areas. In usual clinical presentations we can find paroxysmal hypertension, tachycardia, sweating, and headache. In some cases it will lead to severe cardiovascular complications such as arrhythmias, myocardial infarction, pulmonary oedema, heart failure, and cardiogenic shock [1]. This manuscript adheres to the EQUATOR and CARE guidelines. Written informed consent was obtained from the patient for this publication.
CASE REPORT
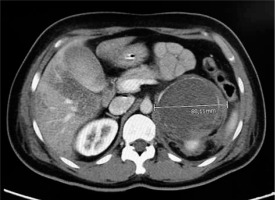
A 48-year-old woman was admitted to the Emergency Department with vomiting, tachycardia, and the following vital signs: respiratory rate 18 breaths min–1, pulse-oximetry 96%, heart rate 140 beats min–1, and blood pressure 163/91 mmHg. She had a history of arterial hypertension unsuccessfully treated with calcium-channel blocker, recent onset type II diabetes mellitus on metformin treatment, episodes of morning vomiting for a few months, and no fever. We ruled out possible causes of exogenous intoxication. During the execution of the blood tests, the patient presented a progressive worsening of the clinical picture with haemodynamic instability towards severe hypotension, marbled skin, and impaired consciousness with GCS 9 (E2V2M5). Arterial blood gas analysis showed severe metabolic acidosis with elevated anion gap: pH 7.17, pCO2 45 mmHg, pO2 115 mmHg, Na+ 137 mmol L–1, K+ 4.8 mmol L–1, HCO3 16.8 mmol L–1, lactate 9 mmol L–1, and Hgb 13.3 g dL–1. ECG showed ST elevation in the anterolateral regions and poor progression of the R wave in the precordial leads. Meanwhile, blood tests showed increase in myoglobin and troponin values. A consulting intensivist performed orotracheal intubation, started mechanical ventilation, and initiated infusion of norepinephrine. The patient was then transferred to the haemodynamic lab for a coronarography. The coronary angiography did not detect stenosing lesions or vasospasms, and an echocardiographic examination showed akinesia of the middle and apex segments of the left ventricle with normokinesia of the apical extremity and of the basal segments with moderate depression of the ejection fraction (EF = 42%) calculated according to Simpson’s formula, right heart sections in normal size, unremarkable pericardium, no valvular defect, and inferior vena cava of normal diame-ter. POCUS examination revealed no signs of pneumothorax. The clinical situation integrated with radiological, instrumental, and laboratory data was compatible with mid-ventricular takotsubo cardiomyopathy. During the angiographic study, further haemodynamic deterioration was seen and cardiac arrest with ROSC after 7 minutes of ALS. Laboratory results included a white blood cell count of 16,500 per μL, a troponin-I level of 77.17 ng mL–1, myoglobin 192.07 ng mL–1, blood glucose 269 mg dL–1, and CK-MB (creatine kinase myocardial band) within normal range. Meanwhile, to ascertain other causes of haemodynamic instability and cardiac arrest, a contrast-enhanced total-body computed tomography (CT) was performed. It excluded pulmonary embolism and acute aortic dissection but highlighted a left-sided adrenal mass with a maximum diameter of 8.8 cm (Figure 1). The patient was admitted to the intensive care unit due to persistent haemodynamic instability despite volume filling by crystalloids (30 mL kg–1 within the first hour) and norepinephrine in continuous infusion. Upon repetition of the echocardiogram, the EF was further reduced to 30%. As a further complication caused by refractory haemodynamic instability, acute kidney injury was established. A continuous infusion therapy of dobutamine (8 g kg–1 min–1) was started because of the cardiogenic shock.
Meanwhile, blood tests showed increased levels of cortisol 76 μg dL–1, adrenaline 306 pg mL–1, metanephri-ne 6620 pg mL–1, normetanephrine 6640 pg mL–1, and renin 291 pg mL–1. Further tests of levels of catecholamines, vanillylmandelic, and homo-vanillic acid in urine were ordered, but the results were not immediately available.
Therefore, given the clinical, radiological, and laboratory situation, the patient started alpha-1-blocking therapy with doxazosin 4 mg per day due to the strong suspicion of pheochromocytoma. After 4 days, despite the pharmacological treatment, haemodynamic instability continued with phases of severe hypotension alternating with phases of refractory hypertension. ECHO showed a moderate improvement in myocardial motion and function, with an increase in the EF up to 45%. After multidisciplinary evaluation with the intensivist, urologist, and endocrinologist, a decision to remove the adrenal mass surgically was made. On the fourth day of intensive care, the patient was brought to the operating room for a videolaparoscopic surgery. The histological examination later confirmed the suspicion of pheochromocytoma with a pheochromocytoma of the adrenal gland scaled score (PASS) < 4 [2, 3].
The ICU stay continued for 10 days postoperatively, until the patient’s clinical condition stabilized, and then she was transferred to the surgical ward. The patient also tested negative for genetic tests on familial syndromes that could lead to the onset of pheochromocytoma.
DISCUSSION
Our patient had an uncommon clinical onset of pheochromocytoma with an endocrinological emergency characterized by haemodynamic instability, cardiogenic shock with tako-tsubo syndrome, and cardiac arrest.
Characteristic of classic takotsubo syndrome is a transient balloon-like modification of the left ventricular apex, visible with imaging techniques such as echocardiography or magnetic resonance, which makes the ventricle take the shape of a basket (tsubo) used by Japanese fishermen for catching octopuses (tako). This is a temporary and reversible cardiomyopathy that occurs more often in post-menopausal women after intense emotional or physical stress, which our patient did not report. It describes a syndrome characterized by symptoms and electrocardiogram signs of acute myocardial injury without coronary stenosis or spasm, in which the heart takes the characteristic shape that gives the syndrome its name [3–5].
An increase in circulating catecholamines is also associated with this syndrome. Proposed mechanisms of cardiac dysfunction include direct toxic effects of catecholamine products on the myocardium, direct catecholamine receptor-mediated effects, coronary vasospasm, and myocardial dysfunction. The apical swelling reflects the high local toxic concentration of cate-cholamines.
Pheochromocytoma presenting as takotsubo syndrome is a known but rare event. It is hypothesized to be related to excessive catecholamine release [4, 5].
Typically, takotsubo syndrome presents with akinesia and ballooning of the apex, but in several reports, as in our presented case, patients with pheochromocytoma-induced cardiomyopathy had severe left ventricular dysfunction with akinetic basal and mid-ventricular segments and hyperkinetic apex, so-called “inverted” takotsubo syndrome. This suggests that there is no single pattern of ventricular dysfunction in these clinical contexts [6–8].
It has been hypothesized that individual variety in the cardiac sympathetic innervation or in the distribution of adrenoceptors could explain the involvement of different parts of the left ventricle [9, 10].
In our case, the combination of acute heart failure with cardiogenic shock, healthy coronary vessels, blood pressure instability, echocardiographic imaging, and elevated levels of catecholamines with a documented adrenal mass supported the diagnostic suspicion of pheochromocytoma-induced takotsubo syndrome.
When our patient was treated by fluid resuscitation and vasoactive drugs, the renal failure and haemodynamic instability did not significantly improve until surgical removal of the adrenal mass, despite the introduction of alpha-blocking agents and a moderate recovery of the EF.
To date, there are no consolidated data on the incidence of pheochromocytoma-induced takotsubo syndrome and the pheochromocytoma remains a rare cause. So, given the lack of large and consolidated studies and the potentially lethal consequences, in consideration of the reversibility of the cardiac function, we would like to point out the importance of a multidisciplinary approach for prompt diagnosis and treatment.
In conclusion, in the presence of recent and rapid onset cardiomyopathy and healthy coronary vessels, the differential diagnosis should focus on, among others, takotsubo syndrome, acute myocarditis, and unrecognized pheochromocytoma.