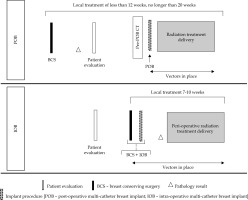
Purpose
Breast conserving surgery (BCS), followed by radiation therapy, is crucial in reducing local recurrence risk for breast cancer patients. This is applicable not only for primary tumors, but also for cases of recurrence after a second conservative treatment [1, 2]. Breast brachytherapy, encompassing accelerated partial breast irradiation (APBI) [3] or as a boost after whole breast radiation therapy (WBRT) [4-6], has been firmly established for selected patients, as recommended by international guidelines [7-10]. After a second conservative treatment, such as accelerated partial breast re-irradiation (APBrl) [11], it is also offered to patients with an ipsilateral breast tumor recurrence (IBTR) as salvage therapy.
Multi-catheter interstitial breast brachytherapy (MIB) emerges as a versatile radiation technique due to its ability to confine the irradiated target volume, thereby reducing organs at risk exposure, minimizing complications, and enhancing cosmetic outcomes [12, 13]. Furthermore, MIB offers a reduction in treatment logistics, typically completed in under 5 days, in the era of normo-fractionation or hypofractionation. Thus, it updates patient logistics [14-17].
The MIB technique is tailored to BCS and brachytherapy timing. There are two distinct methods. The first is post-operative implant procedure, which is followed by a post-operative brachytherapy (POB). The second employs an intra-operative implant procedure, followed by a peri-operative brachytherapy (IOB). This manuscript meticulously analyzed both the techniques, exploring technical, clinical, patient, and logistical nuances from the most relevant published data and expert criteria [3, 18-27]. Through this comprehensive evaluation, breast surgeons and radiation oncologists can gain insights to choose the most suitable procedure for implementation in their clinical practice.
Material and methods
A comprehensive scientific review faced limitations due to the absence of technical aspects referenced in key words or abstract descriptions. These limitations were overcome by selectively extracting relevant series from expert teams in breast brachytherapy. Our analysis focused on comparing experiences of both multi-catheter interstitial breast brachytherapy (MIB) techniques. Additionally, insights from GEC-ESTRO BCWG meetings and expert recommendations enriched our study.
Surgical consideration of breast conserving surgery technique for multi-catheter breast implant
Candidates for MIB primarily target those with small tumors undergoing BCS. APBI with POB ensures excellent oncologic outcomes and cosmetic results [21].
Long-term cosmesis relies heavily on surgical criteria and technique, regardless of radiation technique used. Essential surgical criteria, involving minimal specimen size and discreet incisions (periareolar, axillary, sub-mammary, or lateral chest) to avoid volume depletion or poor cosmesis, are recommended to achieve the optimal cosmesis outcomes [28]. This “scarless” surgery allows a wide subcutaneous dissection over a tumor location and local reconstruction with adjacent breast tissue, which is a very suitable surgical technique for MIB. Unlike other intra-operative radiation techniques, such as intra-operative electron radiotherapy technique (IOERT), MIB adapts to incisions, avoiding extensive scars. Additionally, oncoplastic surgical techniques are not a contraindication for MIB [29]. Whenever the tumor target area is easily identified by breast surgeon and radiation oncologist, together, they can delineate the correct area to implant.
Intra-operative sentinel node biopsy, conducted through the same incision, minimizes scarring [30] and provides crucial information for APBI patients, especially in case of IOB MIB technique. Furthermore, intra-operative pathology analysis of margin status addresses the need for further breast tissue resection [31]. The resection margin measurement, in different directions, determine the surgical cavity to be covered by implant. Precise surgical clip placement is also crucial for accurate target definition. If no clips are inserted in place, the area to be targeted cannot be easily and securely identified, and this can contribute to inaccurate target volume delineation [4, 32, 33]. In conclusion, an efficient collaboration between the radiation oncologist and breast surgeon is essential for MIB success.
General aspects of multi-catheter interstitial breast brachytherapy
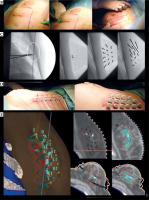
Regardless of clinical indication, MIB prioritizes optimal coverage of tumor bed and clinical target volume (CTV). The implant procedure begins with a “guide needle” at the tumor bed center, with subsequent needles encircling it on planes to ensure complete coverage. A breast template facilitates parallel and geometric catheter placement, while a template-free or free-hand techniques demand expertise [25]. Adhering to Paris system, catheters are positioned at a parallel distance of 1.2 cm to 1.6 cm across distinct planes. This ensures a uniform dose distribution, minimizes high-dose regions, and facilitates a sharp dose fall-off beyond the irradiated zone to protect organs at risk (OARs) [34, 35]. After implant completion, metallic needles are replaced with plastic catheters (Figure 1D). Single-plane implants are generally discouraged to prevent dose inhomogeneity. Pre-operatively measured distances exceeding 10 mm between the tumor and skin prevent skin hyperpigmentation. Special consideration is crucial for deep implants near the chest wall to mitigate risks of neuropathy or costal fractures.
Fig. 1
A case of post-operative brachytherapy (POB) with pre-implant requirements, implant procedure, and dosimetry planning issues. A) Pre-POB CT. The open cavity due to seroma and clips are shown
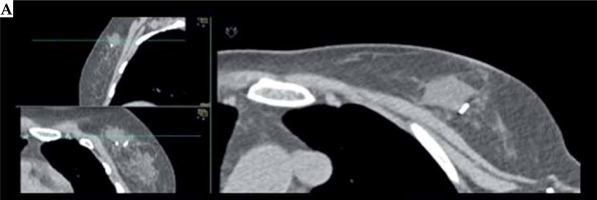
Fig. 1
Cont. B) Implant design of implant direction based on scar. C) X-ray-guided POB procedure. Verification of surgical clips (white circle) and first needle location with MIB template. Metallic vectors are located around surgical clips based on template. D) Metallic vectors are replaced by plastic tubes. E) CT planning with CTV, clips, OARs delineation, catheter reconstruction, and isodose distribution
Post-operative multi-catheter breast brachytherapy
Post-operative brachytherapy is performed post-BCS as a separate surgical procedure, after fulfillment of clinical criteria. Technical and clinical aspects are summarized in Table 1, with an illustrative example shown in Figure 1.
Table 1
Generalities, technical, and clinical aspects of post-operative brachytherapy (POB) and intra-operative brachytherapy (IOB) procedures
Pre-implantation requirement
A pre-implant computed tomography (CT) is conducted a day before brachytherapy. Pre-operative images (mammogram, breast ultrasound, and optional magnetic resonance imaging – MRI), clinical examination, and scar position aid the radiation oncologist in accurately locating the target area. For POB, virtual implantation and planning on pre-implant CT offer crucial guidance for catheter insertion during the procedure. This step enhances the orientation of implant [4], and it is pivotal for the POB procedure. Additionally, it allows for the creation of individualized 3D printing breast templates [36, 37].
Implantation requirements
The POB implant procedure is performed under either local or general anesthesia. Catheters are inserted transcutaneously, via a template-based system, and guided by intra-procedure breast ultrasound or X-ray control to ensure accuracy and visualization of tumor bed clips (Figure 1C). Comprehensive procedural general insights are provided by the GEC-ESTRO guidelines [4].
Organizational aspect
Post-operative brachytherapy should be ideally conducted within 12 weeks after BCS, or at most, 4 weeks after the completion of adjuvant chemotherapy. Non-radiotherapy hospitals are encouraged to facilitate patient referral to specialized breast brachytherapy centers. Figure 2 shows the workflow of organizational process for POB.
Intra-operative multi-catheter breast brachytherapy
After tumor and sentinel node removal, IOB takes place concurrently with BCS, requiring coordinated efforts of radiation oncologists, breast surgeons, and pathologists. Technical and clinical details are outlined in Table 1, with an illustrative example in Figure 3.
Fig. 3
A case of intra-operative brachytherapy (IOB) with pre-implant requirements, implant procedure, and dosimetry planning issues. A) Breast MRI and breast ultrasound, with measures of tumor-skin distance
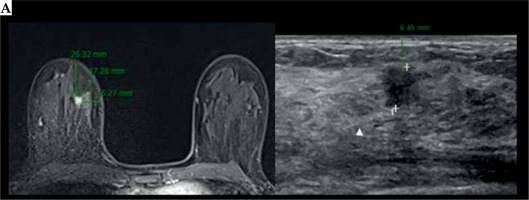
Fig. 3
Cont. B) Pathology macroscopic visualization, margin measurement, and tumor location. C) Intra-operative design of implant procedure of a patient with a peri-areolar incision. Topographical identification of a high-risk area of tumor bed in surgical cavity (solid white line), with clip references (white arrows). The area to be implanted corresponds to a tumor bed plus a 1-2 cm margin, based on visualization of breast specimen and tumor location with margin relationship. D) Final breast conserving surgery with IOB. E) CT planning with CTV, clips, OARs delineation, catheter reconstruction, and isodose distribution
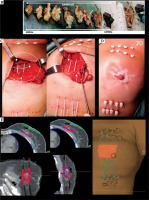
Pre-implantation requirements
Pre-operative images (mammogram, breast ultrasound, and optional breast MRI) offer crucial insights into tumor characteristics (location, size, tumor-skin distance, etc.). They assist in the design of suitable breast brachytherapy procedures. For APBI patients, it is important to note a potential 20-25% rate of unsuitability after implantation; however, these cases can still benefit from an anticipated boost [25, 38]. While a previous planning CT can be considered [4], its utility is limited due to the visualization of tumor bed during the surgical procedure.
Implantation requirements
Post-tumor resection, with precise marking of the surgical specimen is crucial for subsequent intra-operative frozen section histological examination. Tumor visualization within the specimen, along with measuring of macroscopic margin resection, helps in identifying the high-risk area for implantation (Figure 3B). This information guides the brachytherapist in determining the extent of the implant and adjusting its position in the surgical bed. Once the location and depth are established, clips are placed on the surgical bed to indicate tumor boundaries. The implantation area fully encompasses a 2 cm radius margin around the tumor edges, which incorporates the intra-operative macroscopic margin status provided by the pathologist [32]. This ensures proper future CTV delineation and target irradiation [39] (Figure 3E). To minimize geometric disturbances, maintaining a perpendicular implant orientation to the wound closure is recommended. Finally, a drainage system may be retained to prevent seroma, hematoma, or air in CTV, typically removed 24-48 hours after BCS.
Organizational aspect
Local treatment, including surgery and peri-operative adjuvant irradiation, is completed within 7-10 days to confirm the indication (APBI, boost, or APBrl). This is based on definitive pathology results, expected within 48 hours post-BCS. Figure 2 illustrates the workflow of organizational process for POB.
General aspects of planning and treatment delivery
Historically, brachytherapy dose prescriptions relied on reference points, and 2D dose distribution planning was guided by catheter geometries. Modern practice follows 3D image-guided brachytherapy as the gold standard. It incorporates CT images for delineation, planning, and dose calculation. Inverse dose planning and DVH evaluation enhance dose distribution conformity, accuracy, reproducibility, and quality for MIB [34]. Post-implant CT scan validation ensures the accuracy of catheter insertions and implant geometry. The 3D image-guided brachytherapy planning begins with reconstructing catheters and delineating CTV and organs at risk [4] (Figures 1E and 2E).
For CTV delineation, the GEC-ESTRO guidelines determined the process for POB [32, 33]. For IOB, the contour of CTV corresponds to the catheter-based area implanted [39]. Surgeon collaboration can verify clip position. In both the MIB techniques, additional post-implantation needles may enhance coverage.
Second, the prescription dose must cover 90% of CTV, assuring a proper dose homogeneity index (DHI) value. Physicists and radiation oncologists optimize dose delivery for a homogenous dose distribution, following guidelines [4].
Thirdly, once the plan is ready, the treatment begins with implant connection to afterloading machine, typically with high-dose-rate (HDR) sources, such as iridium-192, although PDR can also be used. This procedure takes a few minutes and is repeated 1 to 10 times, depending on the prescribed schedule, at intervals of at least 6 hours. Hospitalization is generally unnecessary. Antibiotic prophylaxis is at the physician discretion, and particularly recommended in IOB. Different protocols can be considered, adapted to each hospital or healthcare system’s patient workflow.
Discussion
Two different MIB techniques are available: POB and IOB. Nevertheless, comprehensive phase III trials comparing POB and IOB remain absent, and retrospective comparative investigations are also lacking. The most relevant studies are summarized in Table 2. In the subsequent sections, we meticulously analyzed both the techniques, exploring dosimetric nuances, clinical outcomes, safety profiles, and toxicities. This analysis incorporated the perspectives of surgeons, radiation oncologists, and patients.
Table 2
Interstitial breast brachytherapy implant relevant studies for post-operative brachytherapy (POB) and intra-operative brachytherapy (IOB)
| Author, year [Ref.] Technique (POB/IOB) | Number of patients (n) | Median follow-up (years) (range) | Early complications rates | Local recurrences (%) (95% CI) | General outcomes OS, DFS, SCC (%) | Toxicity |
|---|---|---|---|---|---|---|
| Arthur et al., 2008 [18] POB | 99 | 7.0 | 1 pt. pneumothorax 12 pts. infection 1 pt. wound dehiscence 1 pt. bleeding | 4.0 | 5-y OS, 93.0 5-y DFS, 87.0 | Acute: G1 (82%), G2 (57%), G3 (3%), G4 (9%) Late: G3 (HDR, 2%; LDR, 9%) |
| Vicini et al., 2019 [46] POB (27%) | 4,216 (APBI, 2,107) | 10.2 | Not reported | 3.9 | 10-y OS, 90.6 10-y DFS 78.1 | Late: G1 (40%), G2 (44%), G3 (10%), G4-5 (< 1%) |
| Polgár et al., 2020 [41] POB | 128 | 17.0 | 1 pt. acute skin adverse effect (6 fx. received instead of 7) | 20-y, 9.6 | 20-y DFS, 79.7 20-y OS, 59.5 | Worse toxicity profile in treated volume from 175 to 330 cm3, no further information |
| Strnad et al., 2016 [3] POB | 274 | 63.0 mts. | Not reported | 5-y IBTR, 2.9 | 5-y OS, 97.0 5-y DFS, 96.0 | Acute: G1-2 skin (6.57%), infection (3.28%), hematoma (2.19%) Late: G1 hyperpigmentation (1.8%), fat necrosis (5.1%), G1-2 telangiectasia (17.2%), G1-2 fibrosis (16.1% and 13.9%), G3 fibrosis (0.4%) |
| Strnad et al., 2023 [21, 48] POB | 1,328 (APBI, 655) | 10.36 | Infection (5%), low-grade intra-operative breast injury (5%), mild hematoma (20%) (grade 1, 6%) | 10-y LRR, 3.51 | 10-y DFS, 84.89 | Late: G1-2 skin RTOG (11%), G3 (1%), G1-2 subcutaneous (47%), G1-2 breast pain (20%) |
| Sato et al., 2013 [60] IOB | 157 | 30.0 mts. (1.7-41 mts.) | Wound breakdown for infection (7.5%) | 3 pts. local failure 2 pts. IBTR 1 pts. node recurrence | Acute: G1 (13.2%) G2 (3.1%) Late: Seroma (1.3%), wound breakdown for infection (6.3%) | |
| Cambeiro et al., 2016 [25] IOB | 88 | 35.5 mts. (8.7-62.4 mts.) | 12 in 10 pts. (11.3%) | 1 pt. contralateral | 3-y LRC, 100.0 | Acute: G0 (57.9%), G1 (36.4%), G2 (5.6%) Late: G0 (32.1%), G1 (57.1%), G2 (10.7%) |
| Sato et al., 2017 [27] IOB | 237 | 50.0 mts. (25.5-83 mts.) | Not reported | 4-y IBTR-FS, 98.9 | 4-y DFS, 97.0 4-y OS, 99.6 | Not reported |
| Hannoun-Lévi et al., 2018 [17] IOB | 26 | 37.2 mts. (35.6-42.3 mts.) | Not reported | 5-y LRFS, 100.0 | 5-y OS, 100.0 5-y CSS, 95.2 | Acute: G1 (75.5%), G2 (22.8%), G3 (4.5%) |
| Hannoun-Lévi et al., 2020 [26] IOB | 26 | 63.0 mts. (60-68 mts.) | Not reported | 5-y LRFS, 100.0 | 5-y OS, 88.5 5-y CSS, 100.0 | Late: G1 (60%), G2 (40%) |
| Kinj et al., 2018 [40] IOB | 44 | 40.0 mts. (36-42 mts.) | Not reported | 3-y LRFS, 100.0 | 3-y SS, 100.0 3-y OS, 93.1 | Acute: G1 (26.7%), G2 (0%), G3 (6.3%) Late: G1 (86.7%) |
| Cozzi et al., 2018 [24] IOB | 59 | 40.0 mts. (1-136 mts.) | Not reported | 3-y OS, 89.0 3-y DFS, 97.0 | Acute: Mastitis (1.6%) Late: Fibrosis G3 (5.6%), fibrosis G4 (18.1%), mastitis (5.5%), hypochromia (14.8%), hyperpigmentation (7.4%), telangiectasis (1.9%) | |
| Morales et al., 2020 [38] IOB | 24 | 100.0 mts. (43-137 mts.) | 4 pts. (hemorrhage, 16%; infection or skin necrosis) Late: 1 pt. fat necrosis (8.3%) | LC, 95.8 EC, 95.8 1 pt. true local recurrence (62.2 mts.) 1 pt. elsewhere (103 mts.) | LRC and DC, 100.0 | Acute: G0 (45.8%), G1 (45.8%), G2 (8.3%) Late: G0 (58.3%), G1 (25%), G2 (16.6%) |
| Morales et al., 2022 [15] IOB | 60 | 27.0 mts. (11-51 mts.) | 11% Bleeding: 4 pts. (6.6%) Wound healing: 2 pts. (3.3%) Late: 5.1% | Local control rate, 100.0 Elsewhere control rate, 98.3 | Loco-regional and distant-control rates, 100.0 | Acute: G0 (88.3%), G1 (11.7%), G2 (0%) Late: G0 (63.3%), G1 (36.7%) |
[i] POB – post-operative multi-catheter breast implant, IOB – intra-operative multi-catheter breast implant, OS – overall survival, DFS – disease-free survival, DC – distant control, LC – local control, EC – elsewhere control, IBTR – ipsilateral breast tumor recurrence, IBTR-FS – ipsilateral breast tumor recurrence-free survival, LRR – local recurrence rate, LRFS – loco-regional-free survival
POB vs. IOB: Technical and dosimetry considerations
Post-operative brachytherapy and IOB share comparable technical aspects, but nuanced differences deserve emphasis regarding target volume accuracy, number of catheters used, and CTV volume delineation. The real-time nature of IOB provides certainty in target identification accuracy [25, 39]. Moreover, IOB series report a lower number of inserted catheters, covering the tumor bed, pioneering the concept of minimally invasive tumor bed implants [38]. In terms of target delineation, POB is based on cavity type (opened or closed) [29, 30], adhering to the published guidelines for consistent criteria. For IOB, a secure CTV delineation approach is the catheter-based methodology advocated by Sato et al. [39]. This has resulted in smaller irradiated volumes in IOB series, reporting median CTV volumes of approximately 30-44 cc, equivalent to 4-5% of breast tissue irradiation [15, 17, 40]. This contrasts with a 81 cc of CTV (range, 7-275 cc) in the GEC-ESTRO phase III trial [3], or a 63 cc (range, 27-120 cc) in the Budapest trial [41] using POB. Additionally, patients with small breasts, where single-plane implantation is mandatory and CTV is therefore reduced, were not recognized as a risk factor for ipsilateral breast tumor recurrence (IBTR), with a 4-year IBTR-free survival of 97.5% [42]. CTV volumes and OARs doses significantly surpass those observed in other radiation techniques, such as IMRT-APBI or CyberKnife [43-45].
While a dosimetric analysis comparing CTV volumes and other dosimetry parameters between POB and IOB has not yet been published, early-term clinical and toxicity outcomes do not reveal substantial and obvious differences. A recent multi-center study reported initial analysis showing that POB and IOB provide equivalent dosimetric and early-term clinical (oncological and toxicity) results, presented at the ASTRO 2023 meeting [19]. See Table 2 and the examples provided in Figures 1 and 3.
POB vs. IOB: Clinical outcome considerations
Pivotal phase III APBI trials, including the Budapest, GEC-ESTRO, and NSABP B-39/ RTOG 0413 trials, have comprehensively validated MIB for APBI. These trials primarily focused on the POB approach, highlighting its safety and efficacy with more than a decade of case follow-up [21, 41, 46]. In contrast, IOB was explored in institutional prospective registered phase I-II studies with shorter follow-ups [24-27, 47].
The Budapest trial reported a 10-year local recurrence (LR) rate of 5.9%, a 10-year disease-free survival (DFS) of 85%, and a 10-year overall survival (OS) of 80% [20]. The GEC-ESTRO group showed a 10-year loco-regional recurrence (LRR) rate of 3.51%, a 10-year DFS of 84.89%, and a 10-year OS of 90.47% [21]. The RTOG 0413 trial revealed a 10-year LC rate of 3.9%, a 10-year DFS of 78.1%, and a 10-year OS of 90.6% for patients undergoing POB [46]. Turning to APBI-IOB, Sato et al. reported an IBTRs-FS rate of 98.9% in 237 patients treated after 50 months, along with a remarkable 4-year OS and DFS of 99.6% [47]. Cambeiro et al. observed a 100% LC rate for 88 patients, with a median follow-up of 38.4 months [25]. Hannoun-Lévi et al. demonstrated the safety of the technique with 26 elderly patients, showing a 5-year LRFS rate of 100% and a 88.5% OS at a 5-year follow-up [26]. Oncological outcomes from IOB studies are presented in Table 2. While the number of patients treated with IOB-APBI is small and long-term oncological outcomes are still pending, no visible differences in oncological outcomes emerge when comparing IOB with POB. Preliminary results from a recent multi-center study presented at the 2023 ASTRO meeting, involving 516 APBI-eligible patients treated with IOB (50.2%) or POB (49.8%), revealed a 4-year cumulative incidence of local and regional recurrence rate of 2% and 1%, respectively, with no statistically significant differences based on the implant procedure [19]. Additional details are provided in Table 2.
POB vs. IOB: Safety and toxicity considerations
The safety of each technique is assessed through complication rates during implantation. POB complications highlighted in the trials are negligible, and may not be exhaustively reported. The GEC-ESTRO trial reported a 5% rate of infection or low-grade breast injury, but up to 20% rate of mild grade 1 hematoma compared with 2.2% in the WBRT arm [48]. In the RTOG 95-17 study, the following infrequent complications were reported: 1 pneumothorax, 12 infections, 1 wound dehiscence, and 1 case of bleeding in 99 patients treated with POB [46]. Notably, Polgar et al.’s extended experience revealed minimal and not clinically relevant early complications for POB [49, 50]. In case of IOB, a rate of around 10% of peri-operative complications was reported, with common issues, including minor bleeding, infection, and wound healing. Hannoun-Lévi et al. reported a 9% complication rate [17], while Cambeiro et al. documented 11.3% complications, including limited hemorrhages, wound healing, seroma, and infection [25]. Additionally, due to limited data availability, complications related to the IOB procedure may sometimes be attributed to post-lumpectomy problems. In conclusion, both the techniques exhibit comparable surgical and treatment-related safety, with no notable differences in complication rates and toxicities. Further details can be compared in Table 2.
POB vs. IOB: Patient considerations
Patient considerations for POB vs. IOB highlight potential advantages and nuances. For older patients, particularly sensitive to the impact of anesthesia on cognitive functions, IOB stands out by avoiding a second anesthesia procedure [24, 51]. However, local anesthesia in POB could be a viable alternative for such cases. Quality of life (QOL) outcomes, when comparing multi-catheter brachytherapy APBI (POB and IOB) with standard WBRT, favors the brachytherapy approach [52-54]. On one hand, IOB’s efficient process within a single-hospital minimizes logistical complexities [55]; yet, on the other hand, POB offers patients flexibility to choose a different radiotherapy center or physician. The choice between IOB and POB may also depend on institutional factors, country regulations, and healthcare system preferences, impacting whether breast brachytherapy patients are treated as in-patients or out-patients. While specific hospitalization is not mandatory, post-procedure care protocols may vary based on institutional norms.
POB vs. IOB: Surgeon considerations
Independent of the technique used (POB or IOB), the implant design is normally adapted by the radiation oncologist to the surgeon’s lumpectomy technique and choice of incision. The surgical approach for IOB may vary from a perpendicular to a periareolar incision aligned with tumor location. Contemporary trends favor oncoplastic procedures that preserve cosmetic outcomes. Notably, the choice of surgical technique does not contraindicate either IOB or POB, as long as implant geometry is assured. Gimeno-Morales et al. [15] demonstrated the compatibility of minimal invasion surgery with IOB, underlining a crucial collaboration between surgeons and brachytherapists to achieve optimal cosmetic results. Evidence supporting POB feasibility with oncoplastic surgery was provided by Roth et al. [29]. To enhance cosmetics and minimize complications, a Japanese study proposed incision relocation to an inconspicuous site, mitigating wound healing issues in peri-operative APBI irradiation [56].
POB vs. IOB: Radiation oncologist considerations
Logistics play a pivotal role in the choice of breast brachytherapy. Figure 2 provides an outline of both the techniques. IOB presents notable advantages by expediting the local treatment process. Standard APBI schedules include 34 Gy in 10 fractions or 32 Gy in 8 fractions, while very accelerated partial breast (vAPBI) schedules further trim treatment duration to 1 or 2 days (18 Gy/1 fx., 16 Gy/1 fx., 6.2 Gy/4 fx., or 7.5 Gy/3 fx.) [15, 16, 26, 57]. Such a reduction supports patient compliance, lowering infection and implant discomfort risks.
Currently, extreme hypofractionation with 5 fractions WBRT [58] is very competitive with MIB vAPBI schedule treatments [15, 16, 26, 57], because no special equipment for brachytherapy is required. Even though breast brachytherapy alleviates long waiting lists, granting patients swifter irradiation access, with potential economic benefits to healthcare systems, this is not well-described in the literature.
A significant disadvantage of IOB to highlight is the number of patients deemed ineligible for APBI post-implantation after receiving definitive pathology results. This ranges around 15-27% [25, 42, 59, 60]. Nonetheless, these patients who have already undergone IOB can receive an anticipated boost, followed by WBRT [25, 38] or catheter explantation. In contrast, POB allows a simple reconsideration of the appropriate adjuvant irradiation technique if the final pathology makes APBI unsuitable. Fortunately, in these cases, the patient would not receive brachytherapy procedure. Further information are presented in Figure 2 and Table 3.
Table 3
Advantages and disadvantages of multi-catheter implant procedures
Conclusions
Breast brachytherapy emerge as a reliable and safe technique for breast cancer treatment encompassing APBI, boost, or APBrI scenarios. While long-term results are yet to establish equivalence in oncological outcomes, both POB and IOB present comparable dosimetry. The early-term toxicity is considered minimal, with no obvious toxicity differences. The choice between POB and IOB depends on the hospital dynamics, logistical considerations, and resource arrangements. These techniques can be deemed equivalent, and are suitable for implementation in comprehensive specialized breast cancer centers.