Introduction
The patch test is considered to be the standard tool for identifying contact allergens. Repeated open application testing (ROAT) is an important adjuvant in the analysis of allergic contact dermatitis. The antigens in the patch test are more concentrated than those in actual use. On the other hand, combinations in actual products might have a synergistic effect in initiating contact allergy [1]. Repeated open application testing usually only uses one antigen at a time and because of the absence of occlusion, irritant reactions and false positives are avoided. In general, they better reflect real life exposure [2]. Repeated open application testing has been demonstrated to be effective in the diagnosis of allergic contact dermatitis to topical corticosteroids [3]. Initial work on ROAT proposed it to be a sensitive method in the diagnosis of allergic contact dermatitis when standard patch tests were negative. It was especially recommended when less common allergens were suspected to be the cause [4]. It has been reported that the lower patch test concentrations eliciting a positive test reaction, the higher the likelihood of a positive ROAT [5].
However, there are different variables in the context of ROAT, which might influence positivity of tests for eliciting contact allergy, like the concentration of the antigen, frequency of application, site and occlusion. The general recommendation is to use the volar aspect of the forearm, twice daily application and a total duration varying from a minimum of 1 week to 4 weeks. The amount of application recommended is 0.1 ml to cover an area of approximately 5 cm2. A positive reaction usually develops in about 4 days. For research studies, the antigens are dispensed using devices like Eppendorf tubes, but these are not practical for routine use. It is often difficult for patients to use ROAT for daily use products, strictly conforming to these directions. Studies have shown that there is a significant variation in the manner in which practitioners use ROAT [1].
Aim
Our study attempts to address ways in which part of the ROAT technique can be standardized – with respect to the area and amount of application and validation of the same through consensus opinion.
Material and methods
Two proposed modifications for the ROAT technique were drafted and the same was discussed with a total of 10 consultant dermatologists. The participants were given four statements and were asked to choose one option for each statement (strongly agree – agree – neutral – disagree – strongly disagree). The four statements were:
the standardization method mentioned for the amount of application seems effective,
the standardization method mentioned for the amount of application is practical,
the standardization method mentioned for the area of application seems effective,
the standardization method mentioned for the area of application is practical.
All the respondents were also asked to give their opinion on possible limitations and problems of the proposed modifications and possible solutions for the same.
The methodology proposed was stated as below.
Extrapolating the fingertip unit to the area of application, about 0.1 ml would cover an area of approximately 4–5 cm2.
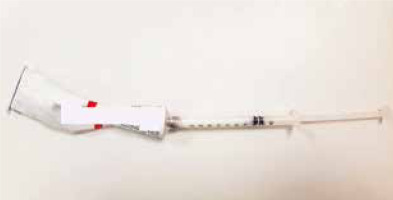
To help the patient use the correct amount of application daily we suggested dispensing the product in easily available 1 cm3 syringes with a detachable needle (cream/ointment and liquid preparations can be easily loaded into these syringes. Two loaded syringes will be enough to cover the recommended 1 week duration. The patient is advised to apply exactly 0.1 ml daily to the test site (Figure 1).
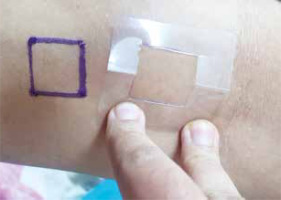
It is also important to ensure that the same area is used for repeat applications. To ensure this we suggested marking an area of approximately 4 cm2 with a stencil, using waterproof surgical markers, over a hairless area on the flexor aspect of the forearm. To further ensure that the markings are not washed away easily, a drop of cyanoacrylate glue is place on the corners of the marked square. The stencil is also given to the patient to further facilitate the application of the cream and if needed the patient can by him/herself reapply any permanent marker ink in case the lines get diluted due to daily washing (Figure 2).
Results
For all the statements, there was a general consensus (strongly agree/agree). For the first statement (the standardization method mentioned for the amount of application seems effective), 5 of the respondents strongly agreed and 5 agreed. For the second statement (the standardization method mentioned for the amount of application is practical) also 5 strongly agreed and 5 agreed. For the third statement (the standardization method mentioned for the area of application seems effective) 2 agreed, while 8 strongly agreed. For the last statement (the standardization method mentioned for the area of application is practical), 3 agreed, 6 strongly agreed and 1 gave a neutral opinion. The points raised in the discussion included the practical difficulty in aspirating thicker ointments into the syringe. Also, the need for another person to assist the patient in remarking the area if required was considered a limitation. Alternative options for marking the area, like the use of a disposable adhesive stencil were discussed. Other suggestions were to encourage patients to take daily photographs of the site to evaluate the evolution of reactions, if any. A patient information leaflet giving details of the procedure and expected reactions was also suggested as a useful tool to help patients.
On the whole, the consensus was that the modifications would be practical and effective and that they would help to simplify and standardize the process better, which in turn would lead to better validity.
Discussion
Repeated open application testing is a very useful adjuvant in testing for allergic contact dermatitis. However standardization of the methodology has been something which needs more study. We focused on two parameters – the area of application and the amount of application.
A survey of 67 American Contact Dermatitis Society members conducted by Brown et al. in 2015 showed that ROAT was less used as compared to patch testing and that there were variations in methodology with respect to frequency of application, duration of application and the anatomical site preferred for application [1].
Hannuksela et al. had postulated that the size of the test area does not affect the results of ROAT [6]. However, Fischer et al., later demonstrated that for the elicitation of nickel allergy, the size of the area was a factor determining the threshold of positivity. The larger the area of application (in effect a larger amount of the antigen) was associated with a lower latency of developing a positive reaction [7, 8]. Besides the exposure dose other factors like the length of time of exposure, may also affect the degree of reactivity [9].
The validation of ROAT and need for clarity regarding some points like the reading of the test reactions have been discussed before by other authors, who have suggested that the results of use tests in general can vary according to factors like the anatomical site and the nature of the skin (diseased or non-diseased). A more standardized method of measuring results was also suggested as a gap to address in the context of ROAT [10]. Grading of the use of bioengineering techniques has been suggested to improve the validity of the test readings. Johansen et al. have previously suggested a more specific scale of evaluation for reading ROAT. In this scale, in addition to an overall clinical impression (on a 5-point scale from strongly positive to negative), four other parameters were specifically evaluated – the percentage of applied area involved, erythema, papules/infiltration and vesicles. The authors also recommended that a detailed morphology of the reactions be given when reporting such results [11]. There is also a gap in identifying differences in ROAT positivity depending on the site of application, especially in the context of diseased vs. non-diseased skin [10]. There have been attempts made to validate algorithms incorporating clinical history with outcomes of ROAT [5].
It is important that patients are given a clear explanation of the rationale of doing the use tests, the methodology and the expected reactions. A short printed leaflet with instructions and details would definitely help patients. We believe that simple standardization techniques like the one we have described will also help in better acceptance as far as patients are concerned.
The small sample size of experts was a limitation in our study. Also the methodology did not follow a proper Delphi technique as the focus was limited. The fact that the methodology was not actually tested in real patients and hence the patient opinion was not incorporated, was the other major limitation.
The modifications we suggested, although preliminary, are simple and practical and could improve the validity of the ROAT. We are planning to pilot the same with real patients to see how it works out in real-life application and consider further modifications accordingly