One of the most common complications of non-obstetric lower abdominal surgery is postoperative pain [1]. In Indonesia, most nonobstetric lower abdominal surgical procedures are performed in regional/rural hospitals with limited resources. Therefore, a strategy should be devised with the aim of reducing postoperative pain that is readily accessible in a limited resource setting. When ma-naged poorly, postoperative pain increases health care workload and hospital expenditure and prolongs the length of stay [2, 3]. Regional anaesthesia is mostly preferred for elective non-obstetric lower abdominal surgery; however, patients commonly prefer general anaesthesia.
The cause of acute lower abdominal postoperative pain is considered multifactorial. Tissue damage induced by surgical trauma triggers the release of inflammatory mediators [4]. Mechanical hyperalgesia accompanying postoperative injuries could contribute to several aspects of postoperative pain. Therefore, antihyperalgesic drugs with membrane stabilizing properties, such as gabapentin, play a role in postoperative pain in combination with antinociceptive drugs to provide a synergistic effect on analgesia [1, 5].
Pre-emptive analgesia is effective in various types of surgery; 60% of studies showed positive effects with decreased pain levels or analgesic consumption after the duration of analgesia [6, 7]. Oral gabapentin is widely used for the management of chronic pain, but no study has been conducted regarding the effectiveness of oral gabapentin as a pre-emptive analgesia in reducing morphine requirements and postoperative pain following nonobstetric lower abdominal surgery. This study was conducted to determine the effectiveness of 600 mg oral gabapentin as a pre-emptive analgesia to reduce postoperative pain and morphine requirements following nonobstetric lower abdominal surgery.
METHODS
This study is a double-blinded randomized clinical trial conducted at Tangerang District Public Hospital as a part of academic health services, Universitas Indonesia. The sample size was calculated using the hypothesis test formula for two proportions based on an expected clinical outcome of 1.2, with a power of 80%, baseline standard deviation 2, and a of 5% [8]. This study was approved by the Ethics and Research Committee of the institution, and all subjects provided consent. We included subjects aged 18–65 years scheduled for elective nonobstetric lower abdominal surgery under gene-ral anaesthesia with an American Society of Anesthesiologists (ASA) physical status of I–II. Subjects were included if body weight fell in the range of ideal body weight ±20% and surgery duration was less than 4 hours. The exclusion criteria were (1) a known history of allergy to the medication used in the study; (2) consumption of analgesia and non-steroidal anti-inflammatory drugs within 12 hours prior to surgery; (3) a history of physical trauma within 4 days before surgery; (4) use of psychiatric medication; (5) diabetes, liver disease or kidney disease; (6) previous gabapentin use before the perioperative period; (7) contraindications to gabapentin, morphine, and paracetamol; (8) pregnancy or breastfeeding; and (9) use of neuraxial block or peripheral nerve block before and during surgery. Subjects were also excluded when (1) the pa-tient wished to withdraw informed consent from research; (2) an allergic reaction was observed perioperatively; (3) a critical condition was observed in surgery; and (4) > 40% estimated blood volume intraoperative bleeding occurred.
Eligible subjects were allocated to the intervention group (600 mg oral) or placebo group by a randomization process using a computer-generated number one day prior to the surgery. The random number was presented in a closed envelope that was opened by the research team two hours prior to surgery. After opening the envelope, the research team administered either 600 mg of gabapentin or a placebo pill to the respective groups. The subjects swallowed the drugs in front of the research team.
In the operating room, standard noninvasive monitoring of the subjects took place. All subjects were co-administered 0.05 mg kg–1 midazolam intravenously and 2 µg kg–1 fentanyl intravenously. Induction of anaesthesia was performed with 2 mg kg–1 propofol intravenously. Endotracheal intubation was facilitated with 0.5 mg kg–1 atracurium. Maintenance of anaesthesia was performed with 1.5–2 vol% sevoflurane, alongside an intermittent bolus of 1 µg kg–1 fentanyl and 0.3 mg kg–1 atracurium as needed. Fentanyl was given with consideration of increased heart rate and blood pressure approximately 10% from baseline values upon the anaesthetist’s discretion after increasing the volatile concentration during surgical manipulation. Atracurium was added if an extra wave between two controlled ventilatory waves on the ventilator monitor and spontaneous movements were observed. Both agents were considered to be added if the surgery was far from completion. Vital signs and the total dose of intraoperative fentanyl were recorded. At the end of the surgery, all subjects were given 0.04 mg kg–1 neostigmine as a neuromuscular block antagonist. The patient was taken to the recovery room and given oxygen using a simple face mask, 10 litres per minute. The first postoperative pain score was assessed in the recovery room by the anaesthetist resident in charge after the patients were deemed fully alert and coherent.
Patient-controlled analgesia (PCA) with intravenous morphine was installed in the recovery room. The patients were previously instructed on how to assess their pain using the visual analogue scale (VAS) score and how to use the device [9]. The measurement scale for pain experienced by patients is in the form of a horizontal 100-mm straight line. On VAS examination, the patient is asked to indicate a point along the line that reflects the degree of pain felt. A ruler in millimetres on the back of the sheet is used to calculate the VAS score. Score 0 = no pain and score 10 = extreme unbearable pain. The patients were also instructed on how to use the PCA device if the VAS score was more than 3. The main settings for the PCA pump were 1 mg of the requested dose with a limit of 5-minute intervals and a maximum dose of 6 mg during an hour. If adequate pain control was not achieved, the requested dose could be increased to 2 mg, and additional morphine doses could be given at the discretion of the doctor in charge. Paracetamol 1 g t.i.d. was used as non-opioid analgesia in this study. Pain was assessed and recorded by the anaesthetist resident in charge, who was not part of the research team and was blinded to the protocol, while in the recovery room, then at 2, 6, 12, and 24 hours postoperatively.
The primary outcome was the cumulative consumption of morphine within 24 hours after surgery. Secondary outcomes were the VAS score at rest and during movement, time of first postoperative rescue analgesia and the incidence of adverse events.
Statistical analysis
Categorical data variables were displayed as percentages, while numerical variables were presented as the mean and standard deviation for normally distributed data or the median and minimum-maximum value for abnormally distributed data. The independent t-test or Mann-Whitney test was performed to study the differences between groups for continuous variables at one time point. Repeated measurements were analysed using a general linear model (GLM) and ANOVA for repeated measurements. A P-value less than 0.05 was considered statistically significant. c2 or Fisher’s exact test was used for statistical analysis of the side effects, with a significance threshold of P < 0.05.
RESULTS
Seventy-two subjects were allocated to two randomized treatment groups, and the distribution of subjects was even for each group. The characteristics of the subjects in the two groups did not significantly differ (Table 1). No patients were excluded or dropped out (Figure 1).
TABLE 1
Basic characteristics of research subjects
The total amount of morphine required in the gabapentin group was significantly lower than that in the control group at every measurement time point (Figure 2; P < 0.001). The VAS scores at rest and during movement were significantly lower in the gabapentin group than in the placebo group at 0, 2 and 24 hours postoperatively (Figure 2; P < 0.05). The VAS scores at 6 and 12 hours postoperatively were similar in both groups.
FIGURE 2
a) First 24-hour postoperative total morphine requirements in the gabapentin and placebo groups. b) Mean VAS score at rest in the first 24 hours postoperatively

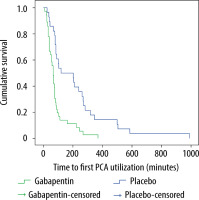
The first time a patient pressed the PCA button reflects the time of first postoperative rescue analgesia. Significant time differences were observed in the first time the patients utilized morphine PCA between the two groups (Table 2; P < 0.001). The Kaplan-Meier curve showed that the placebo group utilized PCA faster than the gabapentin group (Figure 3).
TABLE 2
Time to first postoperative rescue analgesia
| Morphine IV (PCA) | Gabapentin (n = 28) | Placebo (n = 36) | P-value* |
|---|---|---|---|
| First time a PCA requirement (minutes) | 161.5 (25–990) | 67.5 (10–371) | < 0.001 |
Assessment of pre-emptive analgesia adverse events was performed during the first 24 hours after surgery and included complaints of nausea, vomiting, heartburn, and dizziness. Nausea had a greater incidence in the placebo group, but statistical analysis showed no significant difference (Table 3; P = 0.260).
TABLE 3
Overview of adverse events of analgesia in the first 24 hours after surgery
DISCUSSION
Morphine requirements are proportional to the degree of pain. Consequently, subjects with lower degrees of pain will need less [10]. In this study, morphine needs were significantly different, although the degree of postoperative pain in the study population was not severe. Only 28 of the 36 subjects in the gabapentin group pressed the morphine PCA button, while all subjects in the placebo group did.
The results from this study are in line with Turan et al. [11], proving that pre-emptive gabapentin is effective in reducing postoperative opioid consumption in the first 24 hours after surgery. The lower morphine consumption in the gabapentin group can be explained by the role of gabapentin in decreasing the hypersensitivity associated with nerve injury, inflammation, and postoperative pain. Influx inhibition of calcium occurs from the transmission of pain signals from nociceptors through the dorsal horn, ultimately preventing the release of glutamate, substance P, calcitonin gene-related peptide and brain-derived neurotrophic factor. Through the aforementioned mechanisms, the minimization of peripheral sensitization can in turn result in hyperalgesia [12–14].
Another reason for the lower morphine consumption in both groups is the population difference. This study was performed in Indonesians, a part of the Malay race [15], as opposed to the reference study that involved Caucasian subjects. The genetic difference or fewer 118 G allele genes in Malay races demonstrated a lower degree of pain and morphine requirements compared to Caucasian or Indian races. The 118 G allele gene at the µ receptor is more sensitive to electrical and chemical stimuli and is able to influence the opioid mediation process and modulation of pain perception, resulting in increased pain intensity and the use of additional analgesia needs. Other studies also show that racial variations in the broader geographic distribution contribute to the need for additional analgesia 24 hours postoperatively. In this case, Asian populations have a 24% lower need for analgesia than European and American populations [16–19].
On the other hand, Tulandi et al. [15] found no difference in postoperative morphine consumption in the preoperative gabapentin group. The surgical technique chosen for the study subjects was laparoscopic surgery in contrast to open surgery in this study. Postoperative pain in laparoscopic surgery is evidently lower than that in open surgery. Laparoscopic surgery involves less tissue damage, especially on the abdominal wall, which has a rich nerve supply. This correlates well with the mechanism of action of gabapentin in nerve injury.
Gabapentin has a significant ability to reduce acute postoperative pain in the α2δ voltage-dependent calcium channel subunit, which inhibits the excitation of neurotransmitter excitation [20]. Peak serum levels of gabapentin are reached two to three hours after oral intake with an absolute bioavailability of 60%, and the half-life is in the range of 4.8 to 8.7 hours [14, 21], which correlates with the duration of its antihyperalgesic effect. Hence, we observed that this effect was retained as early as the recovery room observation period up until 24 hours postoperatively, as gabapentin was administered two hours prior to the surgery. This fact is supported by Modak et al. [22], who reported that the placebo group had a significantly higher VAS score and that 63% needed morphine in the first hour postoperatively (p < 0.001).
The degree of pain (VAS score) at rest and during movement between the gabapentin and placebo groups significantly differed except at 6 and 12 hours postoperatively. The maximal VAS acquired in this study was approximately 30 mm during rest and approximately 40 mm during movement in the placebo group 24 hours postoperatively, which correlates with mild to moderate degrees of pain. These findings were related to PCA morphine usage provided for the management of acute postoperative pain, which resulted in generally low VAS scores between the two groups.
This finding was in contrast with the results obtained by Bartholdy et al. [23]. The authors found no significant difference in the pain levels after hysterectomy in patients given 800 mg oral gabapentin and placebo; however, gabapentin reduced morphine consumption by 32%. As stated in previous studies, gabapentin requires one to two hours for the antihyperalgesic action to take effect. Bartholdy et al. [23], however, administered gabapentin 30 minutes before surgery. The short duration of surgery (30 minutes) also contributed to the supposed delay of the effect of gabapentin in the reference study [23].
Subjects in the gabapentin groups pressed the PCA morphine button as early as 25 minutes postoperatively compared to 10 minutes in the placebo group, with a significantly different median time between the two groups. Additionally, eight subjects in the gabapentin group did not require extra PCA morphine analgesia and pressed as late as 990 minutes postoperatively. The time when the subjects in the gabapentin group needed rescue analgesia was equivalent to the decrease in gabapentin concentration over time following the kinetic pattern of elimination. In addition, the effect of intravenous 1 g paracetamol analgesia towards the end of surgery has a role in extending the time to press the PCA button.
Few studies have analysed the time to first postoperative rescue analgesia according to the provision of pre-emptive analgesia. Rajhsree et al. [24] found that oral administration of 900 mg gabapentin was able to extend the time up to 104.16 minutes for the first rescue analgesia after laparoscopic cholecystectomy; however, this optimal effect could only be achieved if gabapentin was administered approximately two hours before surgery [24].
The occurrence of nausea in the two groups was not related to intra- or postoperative haemodynamic instability due to bleeding or lack of intravascular fluid. All measurement results of haemodynamic parameters were within normal limits. Despite nausea presenting more in the placebo group, no significant difference was found between the treatment groups. The occurrence of nausea is most likely due to the relatively high consumption of morphine 24 hours postoperatively for the Indonesian population during the occurrence of breakthrough pain, which is consistent with Odom et al. [26], who found that the group with a high degree of pain had a greater degree of nausea than the group with a low degree of pain.
Vomiting can be triggered by noxious stimulation, which affects peripheral receptors activated by the release of local serotonin that will eventually feed back to the brain stem. Autonomic imbalance is also thought to be involved in the mechanism of nausea and vomiting. One cause of the imbalance is a condition that increases sympathetic responses such as surgical stress and postoperative pain [27].
A very recent systematic review and meta-analysis by Verret et al. [28] showed that gabapentinoids unfortunately did not reduce nausea and may be associated with other adverse effects of dizziness and visual disturbances. In our study, the incidence of these adverse effects was not statistically significant.
This study assessed the effects of pre-emptive analgesia on the time to first postoperative rescue analgesia. The time to first rescue analgesia and the total rescue analgesia required are important factors to assess how well the patient tolerates postoperative pain after pre-emptive analgesia before surgery, in addition to studying the antihyperalgesic effects caused by gabapentin pre-emptive analgesia.
This study was conducted in patients undergoing nonobstetric lower abdominal surgery. The broad type of surgery may provide bias, as the type of surgery is not specific. Different procedures exert different trauma to the skin, muscles, fascia, and organs involved in surgery, such as the digestive or reproductive system organs. Each of these is capable of producing a certain degree of pain if nociceptive stimulation is given. This study was not carried out in lower abdominal surgery with a more specific classification.