Purpose
Despite effective screening and vaccination against human papillomavirus, the incidence of cervical cancer in China remains at a relatively high level, with more than 100,000 new cases each year, accounting for nearly 18% of the total new cases worldwide [1]. Radiotherapy can be used not only as means of radical treatment for locally advanced cervical cancer (LACC), but also as auxiliary treatment in early post-operative cervical cancer patients. Concomitant chemoradiotherapy (CCRT), consisting of platinum-based chemotherapy with external-beam radiotherapy (EBRT) followed by brachytherapy, is considered the standard treatment approach and has been widely used in clinical practice. Previous studies reported 5-year overall survival rates ranging from 61.9% to 77.9% in LACC patients [2-4]. Differences of this magnitude have major implications in a number of clinical tasks, such as stratification in trials, matched-pair analysis, decision regarding adjuvant treatment, patient counseling, and comparison of outcomes between centers and studies [5]. Many prognostic factors have been confirmed in cervical cancer patients treated with CCRT, including stage [2, 3], age [6], tumor size [7], lymph node metastasis [2, 4, 8], histological type [8], concurrent chemotherapy [9, 10], serum squamous cell carcinoma antigen (SCC-Ag) [11], overall treatment time (OTT) [12], and anemia [13]. This retrospective study aimed to report our institutional experience of curative radiotherapy in patients with LACC and evaluate prognostic factors.
Material and methods
Patients
Clinical data of 211 patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB2-IIIB cervical cancer treated in our institution between June 2014 and February 2017 were reviewed retrospectively. Inclusion criteria were as follows: 1) Biopsy-diagnosed cervical SCC or adenocarcinoma (AC), 2) Locally advanced disease (FIGO IB2-IIIB), and 3) No previous surgery or external-beam radiation therapy for cervical cancer. Pre-treatment evaluations consisted of complete medical histories, physical examinations, full blood counts, biochemical profiles, serum SCC-Ag levels, pelvic magnetic resonance imaging (MRI) or computed tomography (CT), abdominal CT, and chest X-ray or thoracic CT. Positron emission tomography (PET) was optional. Enlarged lymph nodes with a short diameter ≥ 0.8 cm and/or detected as positive by functional imaging techniques, such as PET-CT or diffusion-weighted MRI, were considered to be clinical lymph node metastasis [8]. The study was approved by the Jilin Province Cancer Hospital Institution Review Board.
Treatment
Treatment comprised of EBRT to the pelvis with concomitant chemotherapy, followed by high-dose-rate (HDR) intra-cavitary (IC) alone or intracavitary/interstitial (IC/IS) brachytherapy based on CT. The treatment field extended from the L4-5 interspace to 3 cm below the most distal vaginal or cervical site of disease. Para-aortic radiotherapy was applied in patients considered at high-risk for para-aortic nodal metastasis at the discretion of treating physician. Clinical target volume (CTV) covered gross tumor volume (GTV), cervix, uterus, parametrium, upper part of the vagina, and drainage area of pelvic lymph nodes. CTV of para-aortic lymph nodes included any enlarged para-aortic lymph nodes plus a 0.5-1 cm margin radially, and vascular structures, such as the aorta and inferior vena cava. CTV plus an 8-10 mm margin was defined as planning target volume (PTV). GTVnd contained all positive regional lymph nodes. Planning gross tumor volume (PGTVnd) expanded GTVnd by a 5 mm margin. The prescribed dose was given as a 1.8-2 Gy fraction daily, 5 days per week, up to a total dose of 45.0-50.4 Gy in 25 to 28 fractions to PTV. For patients with positive regional lymph nodes, 56.0-61.6 Gy was delivered to PGTVnd using concomitant integrated boost technique. Synchronous sensitization chemotherapy regimen consisting of cisplatin (40 mg/m2) was administered once a week for up to 5 weeks, as allowed by patient’s health condition.
HDR-BT was delivered twice a week with an iridium-192 (192Ir) source (Elekta) after a 3rd week of EBRT, if patients were suitable. CT imaging was performed to support real-time treatment plan determination after applicator implantation. All high-risk clinical target volume (HR-CTV), intermediate-risk clinical target volume (IR-CTV), and organs at risk (OARs) were delineated according to GEC-ESTRO and ICRU recommendations [14, 15]. HR-CTV consisted of the whole cervix and residual GTV, which was composed of any manifested residual tumor extension at the time of brachytherapy and residual pathologic tissue as defined by clinical examination and MRI at that point in time, taking into account tumor extent at diagnosis. IR-CTV encompassed either tumor extension at diagnosis or a 1 cm margin around HR-CTV. The planning aim of 24-36 Gy was given to HR-CTV in 4-6 fractions. Absorbed doses to these volumes were converted into a radiobiological equivalent of 2 Gy per fraction (EQD2) and biological effective dose (BED), with an α/β value of 10 for tumors and 3 for OARs. Interstitial needles were used in patients with large tumors and parametrial involvement, especially in those with stage IIIB disease.
After stratifying for nodal status, FIGO stage, tumor load, and other important prognostic factors after completion of CCRT, some patients were administered adjuvant chemotherapy (ACT) with paclitaxel 135-175 mg/m2 D1 and cisplatin 50-70 mg/m2 D1-3, every 21 days in 2-4 cycles.
Follow-up
Our electronic case system could browse relevant information of patients’ previous examinations and treatments, from where the follow-up system exported the lists of entered patients and their telephone numbers. Then, trained professionals followed up them regularly until August 2021, and recorded events about survival and toxicities. All patients were clinically evaluated for tumor response at 3 monthly intervals for the first two years, and biannually for next three years. Radiological assessment with abdomen and chest CT and pelvic MRI was done at a 3rd month follow-up and yearly thereafter, or earlier if clinically indicated.
Statistical analysis
Local control (LC) was selected as the primary end-point and defined as absence of any recurrent or progressive disease in the cervix, parametria, uterine corpus, and vagina. Overall survival, disease-free survival, nodal control, and late morbidity were secondary aims. Overall survival (OS) was defined as absence of death from any cause. Disease-free survival (DFS) was characterized as absence of any disease event or death from any cause. Nodal control (NC) was defined as absence of any recurrent or progressive nodal disease in the pelvic, inguinal, or para-aortic region. Late morbidity was described as any morbidity at 3 months or longer after end of treatment. Primary and secondary outcomes were assessed in all patients with available data. Rectal and bladder toxicity were graded according to EORTC-RTOG late toxicity criteria.
All statistical analyses were performed with SPSS version 19.0 using Kaplan-Meier survival and Cox regression analysis. T-test was applied to compare dose parameters. Time intervals for LC, OS, DFS, and NC were computed from the date of diagnostic biopsy to the date of event or last follow-up.
Results
Patients’ characteristics
The baseline number of the patients was two hundred and fifty, and thirty-nine patients were lost to follow-up (twenty-eight) or incomplete treatment (eleven). The percentage response on tracking was 88.8%. Finally, two hundred and eleven patients were enrolled in the study, and detailed clinical characteristics are summarized in Table 1. The median age of the patients was 53.5 years, and predominance of squamous cell carcinoma (92.4%) was observed. FIGO IIB and IIIB stages were the most common stages (42.2% and 44.1%, respectively). Among the patients, 64% showed bulky tumors (> 4 cm), and 60.2% had lymph-node-negative disease. A serum SCC-Ag level ≥ 30 ng/ml at the time of diagnosis was observed in 28 (13.3%) patients. Precise radiotherapy techniques, intensity-modulated radiation therapy (IMRT), or volumetric modulated arc therapy (VMAT) were delivered in 90% of the patients. Most patients received a dose of 50 Gy or 50.4 Gy in EBRT plus 30 Gy/5 fx. brachytherapy. Only three patients were given a dose of 45 Gy during external irradiation, among them, two cases were IB2 stage and one was IIIB (refusing further external irradiation after 45 Gy/25 fx.). Five patients older than 70 years with IB2-IIB stages received 24 Gy/4 fx. brachytherapy to reduce intestinal irradiation injury. Sixty-six patients, 90% of whom belonged to the group of IC alone, were given 36 Gy/6 fx. brachytherapy to ensure treatment effect. Their common characteristics were: IIIB, size of tumor larger than 4 cm, and poor regression after EBRT. A total of 89.1% (n = 188) of the patients received concurrent platinum-based chemotherapy, and 73.9% (n = 156) were able to tolerate 4 or more cycles. After the end of radiotherapy, 2-4 cycles of adjuvant chemotherapy were completed in eighty-six (40.8%) patients. The mean cumulative dose to 90% of HR-CTV volume (D90) was 89.24 ±5.33 Gy EQD2 (n = 10), and 82.5% (n = 174) of the patients who received ≥ 85 Gy. The mean OARs dose was 77.8 ±8.9 Gy and 81.0 ±9.9 Gy EQD2 (n = 3) to the rectum and bladder, respectively. Combined IC/IS brachytherapy was applied in 31.3% (n = 66) of the patients with persistently extensive disease, and most of them were in stage IIIB.
Table 1
General characteristics of 211 patients
[i] SCC – squamous cell carcinoma, AC – adenocarcinoma, PLNs – pelvic lymph nodes, IMRT – intensity-modulated radiation therapy, VMAT – volumetric modulated arc therapy, 3D-CRT – three-dimensional conformal radiation therapy, IC/IS – intracavitary/interstitial, HR-CTV – high-risk clinical target volume, D90 – dose received by 90% volume, EQD2 – equivalent dose in 2 Gy/fx.
Outcomes and patterns of failure
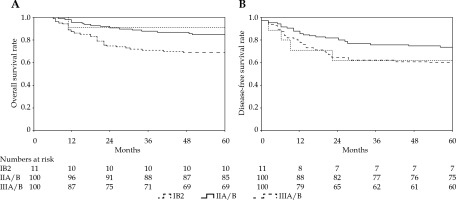
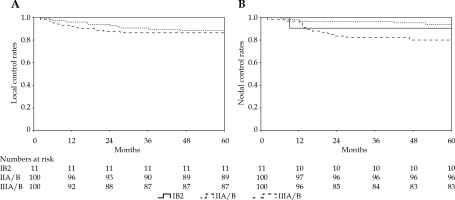
With a median follow-up period of 69 months, 47 (22.3%) patients died, including 44 due to cervical cancer, 2 of heart disease, and 1 of a second primary tumor (colon cancer). A total of 59 (28%) patients experienced treatment failure, namely, 15 (7.1%) with pelvic relapse, 26 (12.3%) with distant metastasis, and 18 (8.5%) with a combination of both. The 5-year LC, OS, DFS, and NC were 89%, 78%, 67%, and 88%, respectively (Table 2, Figures 1, 2). Complete response following treatment as assessed by imaging and gynecological examination was achieved in 192 (91%) patients.
Table 2
Univariate analysis of clinical outcomes
Prognostic factors
As demonstrated in Table 2, the univariate analysis showed that patients with pre-treatment SCC-Ag value ≥ 30 ng/ml received an inferior LC. Pathological type, pre-treatment SCC levels, and FIGO stage were significant prognostic factors for OS. In addition, DFS was also statistically impacted by positive pelvic lymph nodes (PLNs) metastasis. Similarly, PLNs and pre-treatment SCC resulted in a worse NC. Patients with bulky tumors (≥ 4 cm) tended to have poor outcomes in terms of OS and DFS, although there were no significant differences (p = 0.104 and p = 0.119).
Multivariate analysis results are shown in Table 3. The patients with AC had a worse OS (95% CI: 1.020-8.607%, p = 0.046) and DFS (95% CI: 1.321-6.878, p = 0.009) than those with SCC. Positive PLNs were associated with shorter DFS (95% CI: 1.080-3.392, p = 0.026) and NC (95% CI: 1.591-8.429, p = 0.002). The patients with pre-treatment SCC-Ag levels ≥ 30 ng/ml had a worse LC (95% CI: 1.181-6.680, p = 0.020), OS (95% CI: 1.132-5.405, p = 0.023), and DFS (95% CI: 1.033-3.828, p = 0.040) than those with SCC-Ag levels < 30 ng/ml. FIGO stage was an independent prognostic factor for OS (95% CI: 1.222-5.423, p = 0.013).
Table 3
Multivariate analysis of clinical outcomes (not provided in the study)
Role of interstitial brachytherapy in IIIB stage patients
In IIIB stage, combined IC/IS brachytherapy was used in 53% patients, and a dose ≥ 85 Gy was applied in 94.2% of these patients. IC/IS was not associated with OS or LC (p = 0.163 and p = 0.262), while DFS of patients treated with IC/IS diverged from that of the patients treated with IC (p = 0.022). HR-CTV D90 (EQD2, n = 10) in IC vs. IC/IS was 91 ±6 Gy vs. 89 ±3 Gy, respectively (p = 0.188). There were few differences in total BED (p = 0.927) and HR-CTV ≥ 60 cm3 (p = 0.497) between the two groups. OARs’ doses (EQD2, n = 3) were significantly affected by different methods of brachytherapy. The rectum and bladder D2cm3 (EQD2, n = 3) were 7.5 Gy and 7.2 Gy lower, respectively, for IC/IS vs. IC (p = 0.001 for both) (Table 4, Figure 3).
Fig. 3
Local control (A), overall survival (B), and disease-free survival (C) according to different methods of brachytherapy
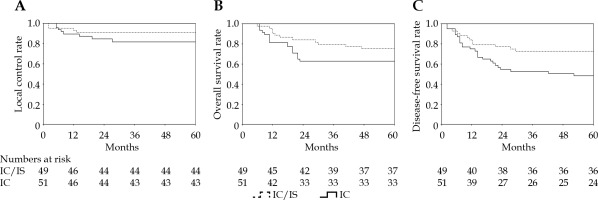
Table 4
Comparison of dosimetry and survival rate in two brachytherapy methods in IIIB stage patients
Toxicity
Vaginal toxicity was not documented for most of the patients. Fifty-two (25.1%) patients developed chronic gastro-intestinal toxicity. Four of them (1.9%) suffered from grade 4 chronic rectal toxicity, including three patients with bowel obstruction and one with recto-vaginal fistula. The D2cc rectum was 88.96 Gy, 90.44 Gy, 76.28 Gy, and 79.25 Gy EQD2 (n = 3). Four (1.9%) patients developed chronic cystitis, and none of them developed ≥ grade 3 bladder toxicity (Table 5).
Discussion
Cervical cancer has a high-rate of treatment failure of approximately 10-20% [16] despite treatment optimization, and pelvic relapse accounts for 70%, of which 50% is accompanied by distant metastases [16, 17]. The present study provided large-scale clinical evidence on the efficacy of CT-based three-dimensional HDR-BT in treating LACC, and emphasized the necessity for evaluating prognostic factors in refining radiation treatment strategies to improve the therapeutic ratio, which can be used as a guide for future clinical work.
In EMBRACE-I study, significant differences were found for DFS and OS using FIGO stage [18]. A retrospective analysis of several GOG trials suggested that FIGO stage was an important factor related to pelvic recurrence [19]. In our study, a significant difference in OS between FIGO stage IIB and IIIB remained, whereas LC, NC, and DFS were not significantly different by stage, which might be related to a stronger association between image-guided brachytherapy or positive pelvic lymph nodes comparing with FIGO stage and disease progression. In the 2018 FIGO classification, imaging was introduced for a new stage IIIC1 and IIIC2 of pelvic and para-aortal nodal disease, respectively [20]. Positive PLNs can reduce OS by 35-40% [21-23]. We found that patients with positive PLNs had significantly worse DFS and NC. Intensification of nodal treatment through a simultaneously integrated boost might be better for disease control [24]. Additionally, there is a room for improvement of para-aortic nodal control, since two-thirds of nodal failures in node-positive patients occurred in the para-aortic region, with majority being outside the elective target volume [25]. Increased use of prophylactic para-aortic irradiation in high-risk node-positive patients will be investigated in our future research.
Previous studies have suggested that non-squamous histology was the strongest prognostic factor for local control [26] and metastatic spread [27]. Moreover, adenocarcinoma leads to a 10-20% lower 5-year survival rate than squamous cell cancer [28]. Our study reached a similar conclusion that patients with adenocarcinoma were more likely to die from the disease and to have disease progression, which might be due to a lower sensitivity of AC to radiotherapy and/or chemotherapy as well as an increased frequency of bulky tumors, leading to a longer time in achieving clinical remission.
Our data also suggested that patients with higher pre-treatment SCC-Ag value had inferior clinical outcomes. Kato and Torigoe first proposed that serum SCC antigen level was an effective marker that could be used to monitor the efficacy of cervical squamous cell carcinoma treatment [29]. On this basis, researchers continued to explore this biomarker and determined that SCC level was closely related to recurrence and death in patients with cervical cancer after CCRT [11, 30]. Lekskul et al. reported a clear correlation between pre-treatment SCC level and tumor size, indirectly indicating the effect of pre-treatment SCC on prognosis [31]. In addition, tumor size showed a strong association with prognosis in some studies [32-34]. In phase III RTOG 90-01 trial, large tumor size (> 5 cm) as a criterion for including patients was addressed [32]. Narayan et al. suggested that tumor volume (≥ 38 ml) was an important risk factor [33]. Beriwal et al. also reported that tumor diameter (> 5 cm) was an important predictor of increased risk of local recurrence [34]. The present study failed to confirm a clear association between tumor size and local control or disease progression, since the local tumor volume was usually within the therapeutic range of radiotherapy, whereas the effect of tumor diameter on survival would be stronger in populations mainly composed of those with stage III/IV disease, with a tumor size often beyond the therapeutic limit of radiotherapy [35].
In contrast to conventional BT, the dose was prescribed in relation to the applicator geometry rather than the anatomy of the tumor. Three-dimensional optimizations could improve tumor target dose coverage while maintaining a safe dose to OARs [36]. Previous studies demonstrated the 3-year LC of 80% for HR-CTV dose < 80 Gy; 88.8% for doses between 80 to 85 Gy and 95.6% for doses ≥ 85 Gy, and this dose-response relationship was more significant in extensive tumors [37, 38]. To reach this threshold of 85 Gy, especially in larger tumors with extensive parametrial infiltration and anatomically unfavorable topography, such as asymmetrical tumor growth, narrow vagina, or vaginal spread of disease, the use of supplemental interstitial needles combined with intra-cavitary applicators might be required [39]. Our clinical outcome showed a decline in overall local control in FIGO stage IIIB of about 6% compared with the value in previously reported EMBRACE-I study, whereas local control in IIIB stage patients receiving interstitial needles in addition to intra-cavitary treatment was close to the benchmark study [18]. The application of IC/IS did not escalate target dose of D90 or affect OS, receiving a significant superiority in DFS and decreased the dose of OARs when compared with IC only.
There was long-term evidence that D2cm3 of the bladder and rectum was associated with urinary and gastro-intestinal side effects [40]. In our investigation, we limited majority of bladder D2cc values to under 90 Gy, since the bladder has a higher tolerance to radiation, and the mean D2cc to the bladder was 81.0 ±9.9 Gy. Only 4 (1.9%) patients suffered from grade 1-2 bladder late side effects, while none experienced grade 3 or above late toxicity. Our mean rectal D2cc was 77.8 ±8.9 Gy. 9.5%, 10.4%, and 1.9% of the patients experienced grade 2, 3, and 4 rectal late side effects, respectively. The potential cause was that to meet the demand of delivered dose for HR-CTV, a fraction of patients had excessive rectal dose (more than 75 Gy), which mainly occurred in stage IIIB patients with IC alone. The application of combined intra-cavitary/interstitial treatment showed safety and feasibility in routine practice, with clinically and statistically significant improvements in the control of disease progression and protection of OARs in LACC.
The advantage of this study was the large cohort size as well as the long follow-up time, which generated mature results. The major limitation was the lack of sufficient patients with stage I and IV disease as well as the study’s retrospective nature. Clinical data of the patients were collected from a single-center, resulting in selection bias and incomplete information. In addition, further investigations with better designs are needed to verify the findings.
Conclusions
In conclusion, EBRT with HDR-BT in patients with IB2-IIIB cervical cancer is efficacious and safe. Identifying related risk factors for prognosis to define risk groups, might be used for intensification of multi-modality treatment in high-risk patients, and reduction of treatment in low-risk patients. For patients with a poor prognosis, more individualized and systemic treatment strategies should be considered for concomitant administration with definitive radiotherapy, to improve the therapeutic ratio. These strategies include MRI-based image-guided brachytherapy, IC/IS brachytherapy with the use of interstitial needles in conjunction with tandem to optimize the radiation target volume, pelvic nodal boost, and extended field radiotherapy.