Aggressive fluid resuscitation can be lifesaving in the initial goal-directed management of critically ill patients admitted to the intensive care unit (ICU) with shock [1]. The purpose of fluid resuscitation in this setting seems clear, but recent studies have demonstrated serious adverse effects when applying this rule to every patient indiscriminately [2]. Fluid overload has been shown to be correlated with morbidity and mortality in ICU patients even after correction for disease severity [3–11].
Fluid resuscitation in patients with shock has the purpose of increasing the stroke volume by increasing the stressed blood volume, which then increases the venous return [1, 12, 13]. This leads to increased cardiac index, but only in patients who are on the ascending limb of the Frank-Starling curve. These are termed ‘fluid responders’, defined by a 10–15% increased stroke volume in response to a fluid challenge [5]. However, critical illness often changes body fluid composition and distribution with a migration from the intracellular to the extracellular, but also from the intravascular to the extravascular space following capillary leak, caused by cytokines and other inflammatory mediators [12]. Hence, the fundamental premise of just giving intravenous fluids may be a gross oversimplification.
The importance of avoiding fluid overload and thus accurate monitoring of a patient’s fluid balance cannot be understated. In a European multicentre study with 3147 patients admitted to the ICU, the authors found a 10% increase in mortality for every 1 litre (L) of positive fluid balance during the first 72 hours of their ICU stay [9].
Because less than 50% of patients with shock respond to fluid resuscitation with an increase in stroke volume, the aggressive administration of fluids serves no purpose in non-responders. It may even have harmful effects, because fluid overload results in severe tissue oedema, compromising oxygen and nutritional delivery, and leading to organ dysfunction and failure [1, 5, 14]. Furthermore, sepsis is associated with generalised endothelial injury and capillary leak [15, 16], which compounds organ dysfunction and volume overload without the desired increase in effective circulating volume [8, 17–19]. Hence, accurate assessment of intravascular volume is imperative, and early use of vasopressors should be considered at the appropriate time to increase the stressed volume whilst avoiding the deleterious effects of excessive fluid administration [20].
To assess for fluid overload, currently used methods include the calculation of fluid inputs and outputs to determine the daily and cumulative fluid balance, measurement of daily weight, monitoring of filling pressures or volumetric preload indices, and assessment of clinical and biochemical signs indicating fluid overload. These methods do not give us an accurate view of a patient’s fluid status, and importantly, do not identify the fluid shifts between different body compartments [21].
Gold standard techniques used to determine fluid composition, such as dual-energy X-ray absorptiometry and isotope dilution, are not feasible at the bedside in ICU patients [4]. Bio-electrical impedance analysis (BIA) presents a potential solution as a non-invasive, practical, and low-cost alternative [15, 22–24]. Recent data have shown its superior value over the classic daily fluid balance for prognostication [25].
The aim of this retrospective, observational study is to assess the prognostic value of fluid overload (FO) measured by BIA in the first week of ICU-stay.
METHODS
Ethical regulations
The study was conducted in accordance with the study protocol, the Declaration of Helsinki, and applicable regulatory requirements. The local Institutional Review Board and Ethics Committee of the Ziekenhuis Netwerk Antwerpen, ZNA Stuivenberg approved the protocol (EC approval number 4737 with insurance policy Ethias 45.313.314). In view of the retrospective nature of the study, which did not require a deviation from standard clinical ICU care, informed consent from the patient or the next of kin was not essential and was waived.
Design
A retrospective, observational cohort study was performed at the Ziekenhuis Netwerk Antwerpen, ZNA Stuivenberg. Data of 101 critically ill patients admitted to the ICU during the study period (from February 2013 until July 2015), who had BIA measurements performed at least once during the first week of their ICU stay, were collected. General patient data such as age, gender, height, and weight were obtained from the medical records. The ICU and hospital admission and discharge dates were recorded, and the APACHE-II (Acute Physiology and Chronic Health Assessment), SAPS-II (Simplified Acute Physiology Score), and SOFA (Sequential Organ Failure Assessment) severity scores were calculated. Cumulative fluid balance was calculated by subtracting the daily outputs from the inputs from admission until the date of BIA measurement. Intra-abdominal pressure (IAP) values were recorded when available. Also, several laboratory results were obtained from the medical records: Hct (haematocrit, %), total protein (mg dL-1), albumin (g L-1), CRP (C-reactive protein, mg dL-1), urea (mg dL-1), osmolality (mmol L-1), glucose (mg dL-1), sodium (Na, mmol L-1), potassium (K, mmoL L-1), and creatinine (mg dL-1). FO was defined as a 5% increment in volume excess (VE) divided by initial body weight.
Bio-electrical impedance analysis measurements
BIA measurements were performed using a Bio-Scan 920-II multi-frequency analyser (Maltron International, Essex, United Kingdom) during the first week of stay (on day 5.1 ± 2). As per the manufacturer’s instructions, 2 electrodes were placed on the wrist and 2 on the ankle, and bioelectrical impedance was measured at 4 frequencies of 5, 50, 100, and 200 kHz in a completely supine position. Total body water (TBW) (L and %), intra- and extracellular water (ICW, ECW) (L and %), fat-free mass (FFM) (kg and %), fat mass (kg and %), volume excess (L), resting metabolic rate (RMR), protein mass (kg), mineral mass (kg), bone mass (kg), muscle mass (kg), glycogen deposits (g), total body calcium (TBCa) (g), and malnutrition index are the BIA parameters that were obtained.
Transpulmonary thermodilution
When available, transpulmonary thermodilution parameters were analysed. Prior to BIA measurement a triplicate transpulmonary thermodilution (TPTDs) was performed to measure indexed extravascular lung water (EVLWI) by pulse contour cardiac output (PiCCO; Pulsion Medical-Systems, Munich, Germany).
Data analysis
Data are expressed as mean with standard deviation (SD) in the case of normal distribution, or as median (with interquartile range) for non-normal distributed parameters. The statistical analysis involved the 2-tailed unpaired Student’s t-test performed in Excel (Microsoft, Redmond, Washington, USA). Mortality rates were obtained for patients and assessed in relation to the presence of FO using different thresholds: 5, 7.5, and 10% FO. For further analysis between FO+ and FO– cohorts a treshold of 5% FO was used. A survival curve was created for the FO+ and the FO– cohorts. A post hoc analysis was performed comprising the prognostic value of TBW, ICW, ECW, ECW/ICW ratio, VE, FFM, RMR, and malnutrition index. Furthermore, we analysed which biochemical parameters were significantly different in the cohort with FO (FO+) vs. those without FO (FO–). A P-value < 0.05 was considered statistically significant. Receiver operating characteristics curve analysis was performed to identify the best predictive threshold for FO. Stepwise multiple logistic regression analysis was performed in order to determine whether FO is an independent predictor for ICU and hospital mortality.
RESULTS
The data collection was performed between February 2013 and July 2015, and 101 patients were included for analysis. A total of 134 BIA measurements were performed.
Demographic data
There were 49 patients in the FO+ group compared to 52 who were FO–. Baseline demographics includ-ing illness severity scores were broadly similar across both groups, and they are summarised in Table 1.
TABLE 1
Demographic data, length of stay (LOS), severity scores, laboratory results, and transpulmonary thermodilution measurements of the study participants, compared between the group with fluid overload (FO+) and the group without fluid overload (FO–). There are no significant differences between the two groups, except for a higher intra-abdominal pressure (IAP), a lower albumin, and a higher capillary leak index in the group with fluid overload
[i] APACHE-II – Acute Physiology and Chronic Health Assessment, BMI – body mass index, CCR – creatinine clearance rate, CLI – capillary leak index, CRP – C-reactive protein, ELVWI – extra-vascular lung water index, GFR – glomerular filtration rate, IAP – intra-abdominal pressure, K – kalium, Na – natrium, Osmol – osmolality, SAPS-II – Simplified Acute Physiology Score, SOFA – Sequential Organ Failure Assessment.
Biochemical analysis
A significant difference was found for albumin levels in FO+ patients in comparison to FO– patients (23 ± 2.9 vs. 25.9 ± 6.5 g L-1; P = 0.006). We observed a statistically significant difference in capillary leak index (CLI, defined as serum CRP divided by serum albumin). Other biochemical measurements did not differ between the two groups (Table 1).
Fluid balance, volume excess, and distribution in body compartments
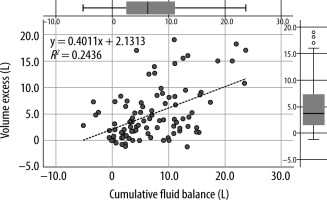
All BIA-derived raw parameters were significantly different in FO+ patients (impedance, phase angle, resistance, reactance, and capacitance) compared to patients in the FO– group (Suppl. Table 1). Table 2 shows the derived parameters; all but one (ICW) were statistically different between the 2 groups. FO+ patients had an average FO of 11.8% and a higher cumulative fluid balance during their ICU stay (8.8 ± 6.7 vs. 5.5 ± 5.4 L; P = 0.009), which was reflected in the derived parameters such as VE (9.9 ± 6.2 vs. 1.5 ± 1.5 L; P < 0.001) and TBW% (63.0 ± 8.7% vs. 52.8 ± 8.1%; P < 0.001). We found a significant correlation between cumulative fluid balance and volume excess (Figure 1).
TABLE 2
Body fluid composition of the study participants measured by BIA. All parameters were statistically different between the two groups, except for ICW. The average fluid overload of FO+ patients was 11.8% with an average volume excess of 9.9 litres. The fluid accumulation was confined strictly to the extracellular compartment, because no difference in the amount of intracellular water (ICW) between the groups could be demonstrated. The extracellular compartment was largely expanded in FO+ patients (27.0 ± 7.3 vs. 19.6 ± 3.7 litres; P < 0.001), reflecting in a high ECW/ICW ratio in the FO+ patients (1.1 ± 0.2 vs. 0.9 ± 0.1; P < 0.001) with a normal ECW/ ICW ratio usually being below 0.8. Nutritional status measured by BIA: FO+ patients had comparable amounts of muscle mass, protein, mineral mass, and glycogen deposits as FO– patients. The RMR in FO+ patients was significantly higher than in FO– patients, leading also to a higher malnutrition index in the FO+ patients
[i] BCM – bio-active cell mass, ECF – extra-cellular fluid, ECM – extra-cellular mass, ECW – extra-cellular water, FB – fluid balance, FFM – fat-free mass, FFMH – fat-free mass hydration, FO – fluid overload, ICW – intra-cellular water, RMR – resting metabolic rate, TBCa – total body calcium, TBK – total body kalium, TBW – total body water, VE – volume excess. *Cumulative FB was calculated from admission until day of BIA measurement.
FIGURE 1
Correlation between (VE, in L) and cumulative fluid balance (in L), calculated in the subgroup of 129 paired measurements. Pearson’s correlation index between VE and cumulative fluid balance was 0.49 (R2 = 0.2436) with a P-value = 0.023
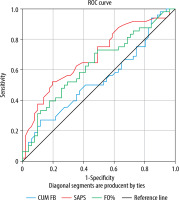
The reported fluid accumulation was confined to the extracellular compartment because no significant difference in absolute water content of the intracellular compartment could be demonstrated (23.9 ± 5.3 vs. 22.5 ± 4.1 L; P = 0.148). In contrast, the extracellular compartment was largely expanded in FO+ patients (27.0 ± 7.3 vs. 19.6 ± 3.7 L; P < 0.001), as illustrated in Figure 2. This is reflected in the ECW/ICW ratio as an inverse relation of relative body fluid distribution was observed in FO+ vs. FO– patients (1.1 ± 0.2 vs. 0.9 ± 0.1; P < 0.001), with a normal ECW/ICW ratio usually being below 0.8. Receiver operating characteristics curve analysis showed that the SAPS-II score had the best predictive value for hospital mortality (area under the curve [AUC] = 0.69, 95% CI: 0.59–0.8) followed by FO (%) (AUC = 0.63, 95% CI: 0.52–0.74) (Figure 3). A 5% threshold for FO had the best sensitivity and specificity for outcome prediction (both around 60%).
FIGURE 2
Fluid distribution in body compartments, expressed in litres (absolute value) for ICU patients with or without fluid overload. Note the strict accumulation in the extracellular compartment versus the comparable amount of fluid in the intracellular compartment. An asterisk “*” indicates a P-value < 0.05

Nutritional status and calorimetrical analysis
FO+ patients had a higher RMR (1708 ± 323 vs. 1589 ± 249 kcal; P = 0.04), but comparable amounts of muscle, protein, mineral mass, and glycogen deposits as patients with no fluid overload (Table 2). Also, patients in the FO+ group had a higher malnutrition index (1 ± 0.2 vs. 0.8 ± 0.1; P < 0.001). Finally, FO+ patients had less fat and more fat-free mass.
Mortality
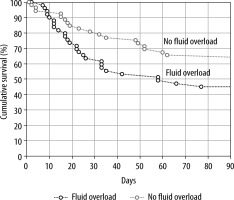
The overall ICU mortality was 39.6% (n = 40) and the hospital mortality was 47.5% (n = 48) for patients in this study. The ICU and hospital mortality were significantly lower in the FO– group compared to the FO+ group. The mortality for the FO– group was, respectively, 31% and 38% in ICU and hospital. When the FO+ group members were cate-gorised into degree of fluid excess (5%, 7.5%, and 10%), there was a stepwise increase in both ICU and hospital mortality. All comparisons between FO– and FO+ patients were significant except for ICU mortality with a 10% FO threshold. This is illustrated in Figure 4. A Kaplan-Meier survival curve is shown in Figure 5; however, the log-rank test did not show statistical significance. Multiple logistic regression corrected for SAPS-II, APACHE-II, SOFA, FO (%), volume excess (L), time of BIA measurement (days), cumulative fluid balance (L), age (years), and BMI, identified SAPS-II score (P = 0.025) and percentage fluid overload (P = 0.015) as independent predictors for ICU mortality. Age (P = 0.015) and percentage fluid overload (P = 0.039) were identified as the sole independent predictors for HOS mortality.
FIGURE 4
ICU and hospital mortality rates in relation to the amount of fluid overload (in %). Fluid overload is expressed as a percentage volume excess from the initial body weight, categorised as no fluid overload (no FO), 5% fluid overload (FO 5%), 7.5 % fluid overload (FO 7.5%), and 10% fluid overload (FO 10%). The ICU and hospital mortality for patients in the FO+ group were significantly higher than in the FO– group (except for ICU mortality when using a 10% threshold). When there was a higher degree of fluid excess in patients their ICU and hospital mortality rates increased stepwise

FIGURE 5
Kaplan-Meier survival curve of ICU-patients, dichotomised by the presence or absence of 5% fluid overload (FO) according to bio-electrical impedance analysisanalysed volume excess. The overall ICU mortality was 39.6% (n = 40) and the hospital mortality was 47.5% (n = 48) for patients in this study. The log rank test showed that the survival curves for patients in the FO– group were not significantly lower than those for the FO+ group
Association between BIA-measured fluid overload, extra-vascular lung water, and abdominal pressure
In the subgroup containing 32 of the 101 patients in which EVLWI was measured by thermodilution, Pearson correlation indices were obtained between EVLWI and BIA-measured ECW, ICW, and TBW. The respective correlation indices were 0.54 for ECW, 0.5 for ICW, and 0.49 for TBW (Suppl. Figure 1). On the total of 134 BIA measurements, IAP was simultaneously registered in 96 measurements. The correlation-index between IAP and ECW, ICW, and TBW was poor – respectively, 0.31, 0.20, and 0.29.
DISCUSSION
The aim of this study was to assess the prognostic value of FO in the first week of ICU-stay, measured by BIA-measurements. Further post hoc analysis was performed on the prognostic value of TBW, ICW and ECW, ECW/ICW ratio, VE, FFM, RMR, and malnutrition index.
Our study found a statistically significant difference in mortality between FO+ and FO– patients. Half of the critically ill patients in the ICU in this study showed significant fluid accumulation in the first week of their ICU stay, and those with FO+ had higher mortality rates. These results confirm previous studies showing a significant association between fluid overload and increased mortality rates [3–11].
Our results show that the group with FO+ had a higher cumulative fluid balance predominantly in the extracellular compartment. A small number of studies have confirmed these findings [26, 27]. A possible explanation is that critically ill patients develop changes in body fluid distribution with migration of fluid from the intravascular to the extravascular compartment [4]. Supporting this theory was the finding that a significant difference was found for albumin levels and CLI between the groups. No previous studies took into account these biochemical measurements. The fact that albumin levels are lower in the FO+ group can probably be linked to leakage outside the vascular space rather than diminished production or increased degradation.
FO+ patients had a higher RMR, but comparable amounts of muscle mass, protein, mineral mass, and glycogen deposits. As a consequence, the FO+ group was more likely to be undernourished, compatible with a higher malnutrition index. This link between malnutrition and fluid overload is confirmed by previous studies [28, 29].
Lastly, the extent of fluid excess (5%, 7.5%, or 10% of initial body weight) had marginal additive effects on mortality. This indicates that a 5% fluid overload seems to have a relatively good predictive value for mortality, defined by volume excess divided by initial body weight. Our method of using VE data in relation to initial body weight in litres per kilogram seems appropriate, since the absolute fluid excess in litres, independently of the patients’ starting weight, would give a less accurate estimation of the patient’s true fluid composition [21].
BIA measurements can be easily performed at the bedside, and based on our results, we recommend its use. As pointed out, daily and cumulative fluid balance do not take into account initial losses that may have occurred. Measurement of daily weight is cumbersome, and daily albumin level measurements are more expensive. BIA measurements in the first week of ICU admission are more accurate in the prediction of mortality than severity scores and less invasive than currently used techniques for determining fluid status, like transpulmonary dye- or thermodilution techniques and pulmonary artery catheterisation.
BIA measurements could help clinicians to guide fluid resuscitation and deresuscitation by giving a daily reliable fluid status including body compartment distribution. As the fluid distribution over the body compartments changes during the course of critical illness, adjustments in drug pharmacokinetics could be made. Further studies are needed to evaluate whether BIA-guided management strategies will improve outcomes.
Our study has several limitations. First, the sample size was small, and the study was retrospective in nature; as such, we were only able to observe associations that do not necessarily mean causation. Second, we did not collect data on the type of fluids administered, and in order to analyse the impact of FO, it would be interesting in future studies to also collect information on the type of fluid, especially if colloids are used. Third, we did not perform BIA measurements on a daily basis but on average on day 5 within the first week of ICU stay. This may have caused a selection bias. However, we feel that because we calculated the cumulative fluid balance from admission until the day of BIA measurement and we found a good correlation between volume excess (VE) and cumulative fluid balance (FB), we were able to obtain reasonable insights regarding the FO+ patients. It must be noted that cumulative fluid balance can be positive while VE may be close to zero, especially in patients with fluid deficit on admission. Therefore, FO defined with measured VE may be more accurate than by using cumulative FB (see Suppl. Figure 2). Fourth, we did not collect information on patient characteristics regarding admission diagnosis, but we did calculate the severity scores instead. Finally, we did not obtain daily measured weight. Future studies need to focus on longitudinal observations looking at daily and cumulative fluid balance, daily weight, and daily volume excess.
CONCLUSIONS
Our study assessed the predictive value of BIA measurements performed during the patients’ first week of ICU stay. A higher mortality rate in FO+ ICU patients was observed. FO was an independent prognostic factor for hospital mortality, because neither APACHE-II, SOFA, nor SAPS-II differed significantly on admission between survivors and non-survivors. Further research is needed to confirm these observations prospectively and to evaluate whether BIA-guided therapy will improve patient outcomes.