Purpose
Unique dose distribution in brachytherapy (BT) allows for appropriate technique as a part of treatment of anal cancer to focally increase the dose to the tumor while sparing healthy tissues [1]. Analysis of the literature shows that brachytherapy, if used as a boost after radiotherapy or chemoradiotherapy, allows for a high probability of local control with acceptable toxicity [2-6]. Apart from the capability of highly focused dose escalation, another advantage of brachytherapy is its ability to spare the uninvolved part of anal canal in order to minimize the risk of long-term morbidity. The majority of published series refer to low-dose-rate brachytherapy (LDR-BT), which delivers a continuous irradiation, and is optimal in terms of radiobiological aspects. Pulsed-dose-rate brachytherapy (PDR-BT) aims at reproducing some radiobiological benefits of LDR-BT (from a radiobiological point of view, PDR is not LDR), and provides the capability of optimization of high-dose-rate brachytherapy (HDR-BT) dosimetry. Moreover, PDR-BT is more convenient in terms of radioprotection, as compared to LDR. Few retrospective series on PDR-BT have been published, including a pilot study of our group with a low number of patients. Those studies suggested that local control would be as good as in LDR-BT [7-10].
Here, we report our experience of PDR-BT use for boosting residual tumor in anal carcinoma, with a prime focus on patients’ outcomes, including functional assessment and health quality perception.
Material and methods
Patients and tumors
A retrospective analysis was conducted among consecutive patients with a histological diagnosis of invasive anal squamous cell carcinoma and treated with a PDR-BT boost in our institution (Gustave Roussy Campus, Villejuif, France) from March 2008 to July 2019. Local ethics committees approved the conduct of this study as well as data collection and analysis (reference Gustave Roussy ethics committee 2021-19). Primary staging included a clinical examination, ultrasound endoscopy, abdominal and pelvic computed tomography (CT) scan, pelvic magnetic resonance imaging (MRI), and 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) for T2-T4 or N+ disease. Tumor staging was carried out according to the American Joint Committee on Cancer 8th edition (Table 1).
Brachytherapy implant
Patients were referred after receiving an external beam radiation therapy (EBRT) irradiating pelvis and inguinal lymph nodes as recommended by the Radiation Therapy Oncology Group, using a 3D conformal EBRT technique, or intensity-modulated radiation therapy with high megavoltage photons [11]. Patients were eligible for PDR-BT if the tumor did not exceed half the circumference of anal canal at diagnosis, and if the thickness of residual tumor did not exceed 5 mm at the time of brachytherapy, according to the clinical assessment. Female patients with an involvement of anterior anal canal were also eligible for BT boost. The procedure for catheters implantation was described elsewhere [10, 12]. It was performed under general anesthesia. The number of needles was chosen to cover the entire residual gross tumor volume, determined by the clinical examination, with a margin of 5 to 10 mm. In case of a doubt, a specialized gastroenterologist performed a trans-rectal endoscopic ultrasound to determine clinical target volume (CTV). The implantation followed Paris system rules (in terms of parallelism and equidistance of needles), and needles were inserted and maintained using a ring template with every centimeter perforated (Papillon template). The implant was sutured to the perineum. An anal dilator was inserted and fixed at the end of the implantation and kept in place until completion of the treatment to spare the uninvolved part of anal mucosa from irradiation.
Treatment planning
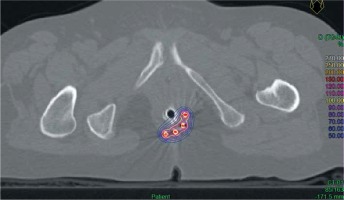
Planning was carried out using a computed tomography (CT) scanner with a slice thickness of 1.5 mm. Axial images were imported into Plato brachytherapy planning system (Nucletron®, Veenendaal, The Netherlands) or Oncentra brachytherapy planning system (Nucletron®). Dwell positions of the implant catheters were digitized on each axial computed tomography slice. Activation length was chosen to properly cover the whole anal canal length of the tumor site. Dose was prescribed to the reference isodose, defined as 85% of the minimal dose-rate isodose between the planes, according to the Paris system. No optimization was performed. Dose distribution is shown in Figure 1.
Fig. 1
Example of a brachytherapy boost for posterolateral residual lesion in a T1 squamous cell carcinoma of the anal canal (axial view). 100% isodose (20 Gy) is shown in yellow. 50% isodose (10 Gy) is shown in blue. Contralateral uninvolved anal canal and vagina are almost totally spared from irradiation
The prescription dose considered EBRT contribution to deliver a minimum dose of 60 Gy to target volume (tumor residuum + 5-10 mm margin). PDR-BT was delivered through continuous hourly pulses, with a dose-rate of 0.4 to 0.6 Gy per hour, 24 hours per day.
Follow-up
The patients had a proctologic examination at 4-6 weeks after BT, then every 4 months for 2 years, every 6 months for 3 years, and then annually. Transrectal endoscopic ultrasound was performed every 6 months for 5 years. Abdominal and pelvic CT scanner or pelvic MRI was done annually for 5 years. Only radiation-related toxicities, including BT-related toxicities were analyzed. Acute toxicity was defined as a complication occurring within the first three months following BT procedure. Late toxicity was defined as a complication occurring more than three months after BT, according to the common terminology criteria for adverse events (CTCAE), version 5.0. Careful biopsy was only performed in case of progressive lesion in endoscopic ultrasound, after a minimum delay of 6 months after brachytherapy. Abdominal perineal amputation was done as a salvage treatment. Failures were classified according to the site of first relapse: local (anorectal or perineal relapse) or distant relapse (lymph nodes or metastases). Complications were assessed from medical records. In addition, the patients were contacted by telephone by a BT-trained technician for updating medical records in terms of toxicities. In addition to this update, a telephone interview was carried out to assess patient health quality perception, with items derived from the EORTC quality of life questionnaire (Appendix).
Statistical analysis
Overall survival (OS), progression-free survival (PFS), local relapse (LR), and distant relapse (DR) rates were estimated using Kaplan-Meier method. Follow-up was calculated from the time between the date of BT to the last follow-up or to the first event. Late digestive, urinary, and gynecological (in females) effects were examined. The total number of late effects were compared with Mann-Whitney test for categorical variables. Categorical variable association analyses were performed with Fisher’s exact test. Median relative tumor shrinkage was estimated with the following formula:
Largest axis at BT time was measured by clinical examination performed under general anesthesia. Anorectal complications were more specifically examined, included rectal bleeding, anal pain, and sphincter dysfunction symptoms. Analyses of the association between the risk of relapse or toxicity and a continuous variable were performed using a logistic regression. All reported p-values were two-sided, and p-values less than 0.05 were considered significant. All analyses were performed using Prism software, version 5.0.
Results
Patients, tumors, and treatments prior to brachytherapy
A total of 42 patients were identified, including 24, 13, and 5 patients with I, II, and III tumor stages, respectively. The median age of patients at the time of BT was 69 years (range, 29 to 85 years). The gender ratio was 0.2 (7 males and 35 females). All patients had received EBRT prior to BT boost. A 3D conformal EBRT technique was used in 23 patients, and an intensity-modulated method was applied in 19 patients. The median EBRT dose to the tumor and the elective nodal volume were 44 Gy (range, 30-54 Gy) and 44 Gy (range, 30.6-54 Gy), respectively. A dose to nodal boost in case of node-positive disease ranged from 50 to 60 Gy. Doses were delivered in fractions of 1.8 to 2 Gy, five fractions per week, except for one patient who received bi-fractionated radiation therapy at a dose of 30 Gy with two daily fractions of 1.5 Gy because of a previous history of post-operative brachytherapy for cervical cancer (60 Gy LDR-BT). The median total duration of external radiotherapy was 32 days (range, 20-77 days). Concomitant chemotherapy was delivered in 16 patients (38%), with II or III tumor stages. Chemotherapy regimens consisted of mitomycin C plus 5-fluorouracil were applied in 10 patients and cisplatin plus 5-fluorouracil in six patients.
For the 5-FU mitomycin C regimen, mitomycin C was administered at a dose of 10 mg/m2 at D1 and D29. 5-FU was administered at a dose of 1000 mg/m2/d over 5 days, on D1 and D29. For the 5-FU cisplatin regimen, cisplatin was administered at a dose of 100 mg/m2 on D1. 5-FU was administered at a dose of 800 mg/m2/d over 5 days, on D1 and D29. For both chemotherapy regimens, chemotherapy was not repeated during brachytherapy.
Brachytherapy characteristics
Brachytherapy was performed within a median time interval of 3.3 weeks (interquartile range, 1.8-4.6 weeks), following EBRT completion. The median overall treatment time calculated from the first EBRT fraction to brachytherapy completion was 55 days (interquartile range, 44-67 days). Fifteen patients had a complete response after EBRT. Among patients with residual disease, the largest median axis diameter of the residual tumor at the time of BT was 15 mm (range, 3-30 mm), and the mean and median residual tumor thickness were 4 mm and 5 mm (range, 1-10 mm), respectively. The implantation of the needles was performed in one plan for all the patients. In two patients, a second brachytherapy procedure was performed to improve needles positioning. The median number of needles was 3 (range, 2-4). The median activation length per needle was 45 mm (range, 25-60 mm). The median number of pulses was 48 (range, 20-80 pulses). The median dose per pulse was 42 cGy (range, 37.50-50 cGy). The median pulse time was 198 s (range, 99-436 s).
The mean and median physical doses delivered through BT were 19.73 Gy and 20 Gy, respectively (range, 10-30 Gy). The mean and median total physical doses delivered (EBRT + BT boost) were 61.94 Gy and 60 Gy (range, 57.84-69.80 Gy). Applying the linear-quadratic model (using an α/β ratio of 10 Gy for tumor target and half-time of 1.5 hours for normal tissues damages repair), the median radiobiologically weighted dose equivalent of 2 Gy/fraction was 19.7 Gy EQD2 for BT boost and 59.7 Gy EQD2 for total dose, including EBRT contribution. The median treated volume (V100% = volume encompassed by the reference isodose) was 9.7 cm3 (range, 4.5-34.28 cm3). The median overdose volume (V200% = volume encompassed by the 200% isodose) was 1.7 cm3 (range, 0.4-7.3 cm3) (Table 2).
Table 2
Characteristics of treatment
Disease control
With a median follow-up of 60.4 months (range, 9.3-127.4 months), a total of five patients (12%) experienced tumor relapse, including three patients (7.1%) with local relapse as the first event and two patients with metastatic failure only (Table 3). In these patients, time intervals from BT to local relapse were 8, 17, and 59 months. Two of these patients had stage II tumor at diagnosis and one had stage III. At BT time, tumor axes were 15-, 15-, and 20-mm. BT doses were 15 Gy, 30 Gy, and 15.2 Gy for a total dose to the tumor of 60 Gy. Time intervals from EBRT to BT were 10, 31, and 41 days. Local relapses were treated with abdominal perineal resection. All the three patients who had a local relapse, subsequently developed metastases. Two of them died of cancer. No patient with stage I disease experienced local relapse.
Table 3
Patterns of recurrences
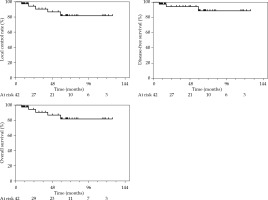
Thirty-eight patients (90%) were alive at last follow-up. Overall local control (LC) rates at 2 and 5 years were 93.9% (95% CI: 77.4-98.5%) and 88.7% (95% CI: 67.4-96.4%), respectively. Estimated disease-free survival probabilities at 2- and 5-year were 93.9% (95% CI: 77.4-98.5%) and 81.2% (95% CI: 59.8-91.9%), respectively. Colostomy-free survival (CFS) rates at 2 and 5 years were 94% (95% CI: 77.5-98.5%) and 88.7% (95% CI: 67.4-96.4%), respectively. Overall survival rates at 2 and 5 years were 100% and 92.1% (95% CI: 71.9-98.0%), respectively. Survival curves are presented in Figure 2.
In univariate analysis, the largest axis of the residual lesion after EBRT was predictive for the overall risk of relapse (p = 0.02). The total tumor dose, initial tumor stage, and time interval between EBRT and brachytherapy were not associated with the risk of relapse (p = 0.7, p = 0.14, and p = 0.43, respectively). The median tumor relative shrinkage was 69% in the whole cohort; 85% among the 39 patients without local relapse and 54% among the three patients who experienced local failure. All relapses, including tumor shrinkage was 85% among patients without relapse vs. 34% among patients with tumor relapse (p = 0.007). Pre-treatment leukocyte count was 4.8 g/l among patients without relapse, vs. 8.9 g/l among patients with a relapse (not significant). No multivariate analysis was performed due to low number of events.
Toxicity
All included patients were analyzed for acute toxicities, which ranged from mild to moderate. One patient experienced a grade 2 anal abscess that required antibiotics and local antiseptic care. Eleven patients (26%) experienced grade 1-2 diarrhea, four patients had a transient and spontaneously resolving acute anal incontinence. No grade 3 or more acute toxicity was reported.
Long-term toxicities assessed among patients free from local relapse (n = 39) are presented in Table 4. No patient required surgery for toxicity. No biopsy-induced ulceration or necrosis was observed in the absence of local recurrence. Grade 1-2 toxicities were as follows: 14 patients (36%) experienced grade 1 perineal telangiectasia, six (15%) and three (8%) patients (21%) had grade 1 and grade 2 diarrhea, respectively. Nine patients had intermittent grade 1 rectal bleeding, and six patients had episodic grade 1 anal pain. A total of six patients had anal sphincter dysfunction symptoms: four had grade 1 fecal incontinence, one had grade 2 symptoms, and one patient had a grade 3 fecal incontinence. Regarding urinary disorders, one patient had intermittent low-level dysuria. Six patients had urinary leakage occurring at most once a week, caused by coughing or effort. Four of these patients reported wearing daily protection. Fourteen patients reported getting up at night to urinate: once (n = 6), twice or three times (n = 7), four or five times (n = 1) per night. Regarding gynecological disorders, 3 of 19 female patients experienced vaginal dryness, and two used lubricants during sexual intercourse. None of the patients complained of vaginal shortening. None of the patients used a vaginal dilator., and no patient had vaginal bleeding.
Table 4
Long-term toxicity according to CTCAE v.5
In details, the only patient with grade 3 incontinence was the one who had an acute anal abscess treated by antibiotics. He was treated for a T1N0 tumor and received 44 Gy EBRT, with three needles implanted. Dose delivered with BT was 20 Gy, for a total dose to the tumor of 64 Gy. Dose per pulse was 50 cGy, which was the highest dose-rate reported in this series. Treated volume was 7.13 cm3. Active length per needle was 45 mm.
Self-rated health perception
A total of 22 patients completed the telephone interview to assess self-rated health perception. For them, the median follow-up from BT completion was 42.2 months (range, 9.3-127.4 months). The median age was 71 years (range, 58-95 years), and included 19 women and 3 men. None of them experienced local or metastatic tumor relapse. Results for self-rated health perception were as follows: very good, good, and poor in 23% (n = 5), 68% (n = 15), and 9% (n = 2), respectively. No correlation was found between good quality of life and occurrence of grade 1+ toxicities (p = 0.1). Twelve patients reported a restriction in daily activities, at high intensity efforts (n = 11) or low intensity effort (n = 1). Both grade 1 and 2 asthenia were reported in seven patients. Four patients had intermittent grade 1 pelvic pain. Nine patients reported psychological effects with intermittent (n = 7) or frequent (n = 2) feeling of sadness.
Factors of toxicity
A total prescription dose ≥ 63 Gy was associated with a higher number of anorectal (including rectal bleeding, anal pain, and sphincter dysfunction symptoms) grade 1+ toxicities (n = 1.5 vs. 0.61, p = 0.02). A BT dose ≥ 20 Gy was associated with a higher incidence of grade 1+ diarrhea (p = 0.02). No correlation was found between the treated volume (V100%) and the risk of any grade 1+ toxicity (p = 0.6), or any specific grade 1+ toxicity (not significant for telangiectasia, fecal urgency, anal pain, diarrhea, fecal incontinence, or rectal bleeding). The hyperdose sleeves volume (volume receiving at least 200% of the reference isodose) was also not a prognostic factor of the risk of complication (p = 0.89).
Discussion
External beam radiation therapy (± concurrent chemotherapy) is the standard treatment for anal canal cancers, allowing a local control of 68% to 87% at 5 years, depending on the stage of tumor [13-15]. The use of BT is an option as a boost modality after EBRT, yielding high local control probability for acceptable toxicity rates. Historically, boosting the anal primary cancer through brachytherapy implied using LDR sources. Papillon et al. [3] reported on a series of 221 patients suitable for sphincter conservation and treated with a split-course regimen, combining external beam radiotherapy at 35 Gy in 15 fractions and interstitial LDR-BT boost to a dose of 15-20 Gy to the initially involved volume, with a short course of 5-fluorouracil and mitomycin C. Tumor control without colostomy was obtained in 80.5% of patients, and 77% of local recurrences occurred in T3-T4 patients. Peiffert et al. [5] conservatively treated 101 patients with a split-course of EBRT with concurrent chemotherapy, followed by LDR-BT after a 2-months-free interval. At 5 years, local control was obtained in 80% of patients and overall survival was 60%. In our series, overall local control rate at 5 years was comparable to data previously published on LDR-BT, confirming the effectiveness of PDR-BT in selected patients, with mainly I-II tumor stages and those who responded well to EBRT.
Pulsed-dose-rate brachytherapy was developed to replace iridium-192 wires. A recent systematic literature review analyzed the results of 10 retrospective studies reporting on BT boost, including three studies using PDR technology [16]. Median 5-year estimated LC, CFS, DFS, and OS rates were 78.6%, 76.1%, 75.8%, and 69.4%, respectively. PDR-BT data were less available due to more recent use (Table 5). Only one of three series using PDR-BT (47 patients with predominantly advanced tumors, as only 44.7% were stage T1-T2), reported outcomes at 5 years (LC = 75.0%, CFS = 76.1%, DFS = 75.0%, OS = 65.0%) [17]. The second study involving 71 patients, reported a 2-year CSF of 89% [7], whereas in the third one, 5-year LC rate was 78.6% for 209 patients treated with BT boost, and included 58 patients receiving PDR-BT. This series did not differentiate outcomes between PDR-BT and LDR-BT [18]. Recently, a large retrospective study on PDR-BT boost after EBRT in more than 120 patients was published. With a median follow-up of 71 months and with 51.2% stages T1-T2 tumors, local control rate at 5 years was 81.7% [9]. In our retrospective analysis on 42 patients with T1-T2 tumors treated with EBRT followed by PDR-BT boost, local control was almost 94% at 2 years and 89% at 5 years. These results compare rather favorably with those from the literature in terms of local control after PDR-BT. This is probably a consequence of patients’ selection, as we mainly included patients at an early stage of the disease. However, our results also compare favorably with published data that used EBRT boost plus concomitant chemotherapy for low-stage tumors. In the Trans-Tasman Radiation Oncology Group (TROG)-9902 trial, local control rate and colostomy-free survival probability at 4 years for patients with T1-T2 tumors treated with EBRT (50.4 Gy to the anal canal and 36 Gy to the pelvis) and with 5FU-mitomycin C, were 82% and 85%, respectively [19]. While two thirds of our patients did not receive concomitant chemotherapy, we may hypothesize that dose escalation afforded by brachytherapy may yield similar local control probability as a strategy based on chemoradiation with lower EBRT doses. Retrospective data evaluating boost modality suggested that BT was superior to EBRT boost when overall treatment time was < 80 days [20]. In our series, the median interval time between EBRT and BT boost was approximately 3 weeks, which was quite a long time in relation with the fact that various patients were referred for BT boost after receiving EBRT in another center.
Table 5
Retrospective series on pulsed-dose-rate brachytherapy
| Authors (reference) | Number of patients | Stage (%) | EBRT dose (Gy)/number of fractions, volumes | Chemotherapy | BT boost median total dose (range), technique | Outcomes | Late toxicity (%) |
|---|---|---|---|---|---|---|---|
| Gryc et al. [17] | 47 | T1: 8.0 T2: 36.2 T3: 38.3 T4: 14.9 N0: 68.9 N1: 11.1 N2: 10.0 N3: 6.8 | 50.4 Gy/28 boost (T1): 5.4 Gy; boost (≥ T2): 9 Gy, AR + PN + IN | 5-FU + MMC: 93% 5-FU + CDDP: 2.3% 5-FU: 2.3% | 15.5 Gy (8-35.8), PDR | 5 years: LC: 75% CSF: 66.1% DFS: 75% OS: 65% | G3-4: proctitis: 16.0; diarrhea: 3.0 |
| Bruna et al. [7] | 71 | T1: 19.7 T2: 57.7 T3: 21.1 T4: 1.4 N0: 73.2 N1: 18.3 N2: 4.2 N3: 4.2 | Mean dose: 45.5 Gy/25; AR + PN + IN (24%) | 5-FU + CDDP: 66.1% | 17.8 Gy (10-25), PDR | 2 years: LC: 91% CSF: 89% DFS: 91% OS: 90% | G3 toxicity (pain, bleeding, fecal incontinence, or necrosis): 14.0, G4 radionecrosis: 2.8 |
| Lestrade et al. [18] | 219 | T1: 12.4 T2: 46.9 T3: 38.8 T4: 2.4 N0: 72.3 N1: 17.2 N2: 7.2 N3: 3.3 | 45 Gy/25, AR + PN + IN (19.0%) | 5-FU + CDDP: 49.7% 5-FU + MMC: 14.8% | 18 Gy (10-31.7) LDR (72.2%)/PDR (27.8%) | 5 years: LC: 78.6% CSF: 79.4% DFS: 80.9% OS: 69.4% | G3-4 anorectal toxicity: 6.3 |
| Arcelli et al. [9] | 123 | T1: 14.6 T2: 36.6 T3: 33.3 T4: 15.4 N0: 56.9 N1: 22.8 N2: 10.5 N3: 9.8 | 45 Gy/25 | 5-FU + MMC: 94% | 20 Gy (13-25) | 5 years: LC: 81.7% CSF: 62.3% DFS: 92.3% OS: 74.0% | G4 anorectal toxicity: 4.9 |
In addition to the possibility to apply focal dose escalation, one advantage of brachytherapy is to spare the non-involved part of the anal canal. In our series, low levels of acute and chronic toxicity were observed. No acute grade 3+ toxicity was noted, and only one severe late morbidity occurred (fecal incontinence). No surgery or colostomy was indicated due to toxicity. Other authors reported 14% of grade 3+ chronic anal toxicities and 2.8% of grade 4 radionecrosis after PDR-BT boost [7]. With 5-year median follow-up, we observed much less chronic toxicities. This may be explained by a smaller volume of treatment, limited to the residual tumor as well as proper patients’ selection. Furthermore, only patients with good response were included, and only one brachytherapy plane (which is enough to treat tumors less than 5 mm thick) was used. Another explanation could be that our median dose-rate did not exceed 0.5 Gy per hour, though such correlation between dose-rate and chronic toxicity was not demonstrated in anal canal cancer. We observed an association between total dose to the tumor and late toxicities, in line with previous data from Peiffert et al. [5], who reported that a brachytherapy dose > 20 Gy was associated with increased late severe complications [5].
A limitation of this study is the heterogeneity of external radiotherapy and chemotherapy protocols due to the fact that patients were referred only for brachytherapy boost, after receiving radiotherapy in various centers. A strength and originality of this study is the evaluation of patients’ health quality perception. More than half of the patients responded to the survey allowing a detailed analysis of delayed toxicity, including gynecological morbidity. Systematic collection of toxicities by questionnaire should therefore be encouraged in daily clinical activities. More than 90% of the patients reported good or very good quality of life, suggesting excellent functional results of this technique. Furthermore, we observed that tumor shrinkage at the time of BT was correlated with probability of a relapse. Such data had been shown in larger cohorts with locally advanced cervical cancer patients who shared some histopathological similarities with anal cancer [21]. This suggests that tumor response on EBRT could be an indicator for a treatment intensification through systemic approaches or dose escalation strategies, which still need to be examined. In the same way, pre-treatment leukocytosis could be relevant for identifying patients at a high-risk of relapse. In our series, the pre-treatment leukocyte count was 4.8 g/l among patients without relapse versus 8.9 g/l in patients with a relapse. The difference was not significant, possibly due to insufficient statistical power (10 patients receiving EBRT in another center without pre-treatment leucocyte count). However, a larger study suggested an effect in various squamous cell carcinoma types, including anal cancer [22-24].
Conclusions
With almost 5 years median follow-up, this study confirms that an approach based on PDR-BT to boost the tumor bed is effective and safe in this indication for selected patients with mainly stage I tumors. Local control rate was comparable with historical data on LDR-BT. Morbidity profile was favorable, possibly as a consequence of accurate patients’ selection and technique used (one implant plane and small treated volumes). Most patients reported good or very good quality of life. Tumor shrinkage was associated with more favorable outcome, suggesting the need for further systemic intensification or dose escalation in patients with poor tumor response. In this context, BT is a good technique to focally increase the dose, provided that patients’ selection criteria and implant rules are carefully followed.