Purpose
Cervical cancer is the fourth most common cancer in women worldwide and is the leading cause of cancer-related death for women in Eastern, Western, Middle, and Southern Africa [1]. In the U.S., the cases of cervical cancer in young females have decreased after the use of human papilloma virus vaccine [2]. The estimated number of cervical cancer patients in 2015 was approximately 12,900 cases [3].
In locally advanced cases of cervical cancer, chemotherapy combined with radiation therapy (RT) remains the current standard of care [4]. External RT and intracavitary brachytherapy delivered via tandem and ovoids (T+O) or tandem and ring, have long been the mainstay of the treatment regimen [5]. Both the above-mentioned RT modalities are generally recommended to maximize local and regional tumor control, and studies have shown decreased survival rates in patients where the brachytherapy is omitted [5-8].
Historically, based on the Manchester system, the location at which the prescription dose of brachytherapy radiation was defined, is known as point “A” located 2 cm superior to the vaginal fornices along the tandem and 2 cm perpendicularly lateral to the tandem [9]. This point also was commonly thought to describe where the ureters cross the uterine artery, which was found to be a point that limited toxicities due to findings of necrosis at the paracervical vessels, making it vulnerable to potential changes in dosimetry [10]. Studies have shown that there is a positive correlation between bladder dose, rectal dose, urinary bladder, and rectal complications, and severity of complications [11]. Therefore, it is vital to weigh the cost-benefit ratio of achieving a high-rate of cure and RT complications, which may at times significantly affect quality of life.
To limit the RT exposure to organs at risk (OARs), the current practice is to insert lubricated strips of gauze or other packing material into the space between the applicator and the anterior and posterior vaginal walls. This fixes the applicator and displaces the bladder and rectum anteriorly and posteriorly, respectively, thus reducing the overall dose delivered to these organs [12].
Malaker et al. [13] described a technique to reduce urinary bladder dose without compromising dose to the cervix by placing an inflated Foley catheter balloon between tandem source and base of the bladder. Eng et al. [14] developed another technique, in which an uninflated Foley balloon was inserted transvaginally above and below the tandem flange and then secured in place with vaginal packing. In this study, there was an average dose reduction of 7.2% and 9.3% to D0.1cc of the bladder and rectum, respectively [14]. Alternatively, for patients with smaller volume vaginal cavities, Rai et al. [15] described a method, in which a bladder-rectum spacer balloon, a disposable latex balloon abutting the bladder and rectum and adjoined at the middle, demonstrated statistically significant dose reductions to the rectum compared to gauze packing technique.
At our center, the T+O applicator used to deliver HDR brachytherapy is usually positioned with vaginal immobilization balloons anteriorly and posteriorly, to displace the bladder and rectum away from the radiation source. The T+O applicator and balloons were left in situ overnight with the patient on strict bed rest, to deliver 3 fractions of brachytherapy. Preliminary data have shown minimal change in immobilization balloon volume while in situ overnight [16]. This is the first study to our knowledge looking at the overnight change of these balloons. In this study, we aimed to show that cervical balloon use for patients being treated with HDR T+O brachytherapy is safe and reproducible for planning and treatment delivery, when left in situ overnight for treatments given on sequential days.
Material and methods
Patients
This retrospective study was approved by our institutional review board (IRB). Eligible patients included females over 18 years of age with locally advanced cervical cancer, undergoing T+O brachytherapy delivery on sequential days. This technique was adopted as our program changed from low-dose-rate cesium-137 to HDR iridium-192, and continued to serve patients who traveled from long distances or rural regions for their brachytherapy in an academic setting.
We reviewed 44 CT simulations of 22 paired treatments in 13 patients. The anterior balloon deflated in one of these paired treatments and was omitted from data analysis, leaving 42 CT simulations of 21 paired treatments in 13 patients.
Procedure
The T+O Fletcher suit applicator (Varian Medical Systems, Palo Alto, CA, USA) was placed in the operating room under general anesthesia. Two immobilization balloons (Radiadyne Alatus, Latham, NY, USA) were then placed, with one arranged anteriorly between the ovoids and bladder, and one placed posteriorly between the ovoids and rectum. The balloons were then inflated in 10 cc of air increments up to 40 cc as patient anatomy allowed with continual checking of positioning of the balloons during inflation. A CT simulation (sim 1) was then performed in the immediate post-op period to determine the positioning of T+O and balloons, allowing for dosimetric planning of the HDR treatments. Prior to sim 1, the Foley was clamped and the bladder was instilled with 30 cc of normal saline and contrast. After contouring and treatment planning (Eclipse BrachyVision version 11.0.47, Varian Medical Systems, Palo Alto, CA, USA), the plan was evaluated and approved by the treating physician and medical physicist. The patients then received their first of 3 brachytherapy treatments over 2 sequential days.
After delivery of the first fraction, the patient stayed in the hospital overnight with the T+O applicator and balloons in situ. A second CT simulation (sim 2) was performed the next morning prior to the second fraction, to ensure that no major movement of the applicator or balloons had occurred. Prior to sim 2, 30 cc of normal saline was instilled into the bladder. The patient received the second fraction, and after the third fraction (all fractions were minimum 6 hours apart), the T+O applicator and balloons were removed and the patient discharged. The patient returned a week later to receive a second T+O implant, with again 3 fractions of HDR separated by a minimum of 6 hours, with fractions 2 and 3 being delivered the following day of implant. Each patient, therefore, had two T+O implants, with a total of 4 CT sim scans (2 × sim 1 and 2 × sim 2) and 6 HDR fractions, which were available for analysis.
Dosimetry
The paired simulations (sim 1 and sim 2) were accessed retrospectively, and primary outcomes including measurements of applicator positioning and balloon volumes were obtained. The distances from the tandem to the pubic symphysis and both balloons to stable bony landmarks, including the pubic symphysis, right, and left femoral heads, were measured on both paired scans, and the overnight change was calculated. Comparisons of the volumes of the anterior and posterior balloons were also made between the first and second CT simulations for the same T+O implant to evaluate for volume loss overnight.
Sim 2 underwent retrospective dosimetric planning, and radiation to OARs (bladder and rectum) were measured in D2cc, D1cc, D0.1cc, and D0.03cc. These values were then compared to the initial values calculated from the sim 1 treatment plan.
Statistics
The mean difference in overnight change of the balloon volumes and bony landmark measurements for the 21 treatments were reported with standard deviation, and statistical significance was calculated comparing both means using Mann-Whitney U test. Statistical significance was considered p < 0.05.
Results
Balloon volumes
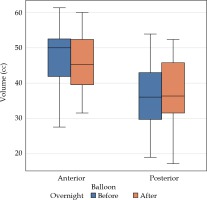
The measurements of the balloon volumes were taken before the first fraction (sim 1) and second fraction (sim 2) to ascertain overnight change (Table 1, Figure 1). There was no statistically significant difference in volumes for the posterior or anterior balloon.
Table 1
Mean volumes of anterior and posterior balloons with mean difference and percent change with standard deviation after balloons left in situ overnight
Balloon positioning
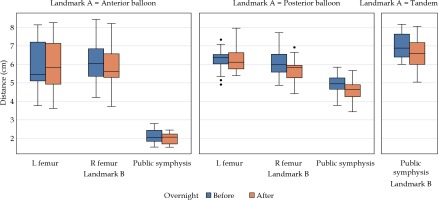
The change in distance from balloons and tandem to bony landmarks was calculated from the paired CT scans (Table 2, Figure 2). The anterior balloon showed no significant difference in distance from the right and left femoral heads or from the pubic symphysis. The posterior balloon had no significant difference in distance from the left femoral head, but there was a significant difference from the pubic symphysis and right femoral head. Differences from the posterior balloon to the pubic symphysis and right femoral head were –0.29 ±0.46 cm (p = 0.03) and –0.32 ±0.50 cm (p = 0.01), respectively. The tandem to the pubic symphysis also showed a significant difference, with a difference of –0.37 ±0.39 cm (p = 0.002).
Table 2
Mean distances between balloons, tandem, and bony landmarks from before (sim 1) and after (sim 2) overnight stay in the hospital, mean difference in distances from sim 1 and sim 2 overnight, and percent change with standard deviation. Bolded numbers indicate statistical significance with Mann-Whitney U test
Fig. 2
Distances between bony landmarks and balloons. A box-and-whisker plot comparison of distances between bony landmarks and anterior balloon, posterior balloon, and tandem with overnight change in distances between balloons and bone (femur indicates femoral head), with balloons and T+O applicator in situ
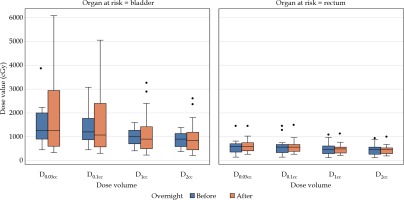
Dosimetric changes
After analysis of the data of the 21 paired scans with balloons remaining inflated in situ, there was no significant differences in the bladder and rectum radiation doses (Table 3, Figure 3).
Table 3
Mean radiation dose to organs at risk at first dose (sim 1) and second dose (sim 2), mean difference in radiation dose between sim 1 and sim 2, and percent change with standard deviation
Discussion
Brachytherapy is an important tool in the definitive treatment of cervical cancer [5, 6]. Patients in rural areas who live a significant distance away from a brachytherapy center, face inconvenience with multiple procedures for T+O HDR brachytherapy delivery. Brachytherapy is a highly specialized treatment that can be difficult to access in rural parts of high-income countries, particularly for older women [17], and also in low- and middle-income countries, where up to 90% of cervical cancer patients may not have access to brachytherapy [18].
For many patients though, there are numerous access barriers to treatment, such as lack of transportation and support [19, 20]. Treatment over sequential days after completion of chemoradiation to the pelvis, allows patients to expedite their overall brachytherapy treatment during an overnight stay at the hospital, thus easing a considerable travel burden for rural patients coming to a brachytherapy center.
There are numerous ways to displace OARs during brachytherapy procedures, including traditional gauze packing, balloons, and retractor blades [12]. Evaluating the overnight variability of these devices is important for dosimetric reasons and disease control, as Viswanathan et al. [21] showing that variability in brachytherapy placement may decrease disease control. As air-filled balloons are an increasingly common packing method [22], it is important to understand the implications of overnight movements and intracavitary pressures on the balloons and T+O applicator. Moreover, it is crucial to ensure that any resultant movements do not compromise the dose and cause potential complications to the bladder and rectum.
The purpose of this study was to assess whether overnight shifting and external pressure (forces external to the balloon, such as applied by body tissues, including the musculature of the vagina that could change the shape, position, or cause deflation of the balloon) while the patient had the T+O and balloons in situ for over 24 hours, resulted in significant changes in balloon dimensions, balloon positioning, and T+O positioning. There were no statistically significant changes in the volume of either the anterior or posterior balloons overnight. Positioning was measured in relation to unchanging bony landmarks. There were no significant changes found in the positioning of the anterior balloon. The mean changes in position of the posterior balloon and tandem were below 0.5 cm, and our data did not show any resultant increase in the bladder or rectum dose between CT sim 1 and CT sim 2. Although the distance between the posterior balloon and pubic symphysis did change significantly overnight in relation to certain bony landmarks, this did not appear to result in a dosimetric effect. Presumably, the target and OARs moved with the applicator/balloons, and so did not alter the radiation dose to those structures.
Despite potential human error with contouring of balloons and measurements, this data supports the use of vaginal immobilization balloons as a potential alternative to gauze packing for selected patients’ populations, showing minimal or no adverse positioning or radiation effects from keeping the T+O applicator in situ overnight.
Conclusions
While this study is limited in its capacity as a retrospective analysis of patients, in whom this treatment was performed, given the therapy’s potential logistical benefit, this data merits further prospective study of the sequential day treatment regimen using vaginal immobilization balloons. These data can add to our understanding of alternative brachytherapy techniques to better serve our patients.