A 35-year-old female patient was previously diagnosed in 2015 as a case of systemic lupus erythematosus (SLE) by internal medicine and rheumatology departments. The patient was treated with corticosteroids (prednisone; 20 mg/day) and immunomodulators (azathioprine: 100 mg/day) with satisfactory results.
The patient presented to our Dermatology Clinic in November 2019 with a skin rash formed of erythematous-maculopapular lesions and targetoid-like lesions on the trunk and extremities, as well as on the face (Figure 1 A).
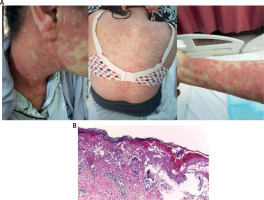
Figure 1
Dusky red maculopapular and plaques with targetoid like lesions in face, trunk and right forearm (A). Hyperkeratosis, acanthosis, necrotic keratinocytes, vacuolar degeneration at basal cell layer. Papillary dermal edema and superficial perivascular inflammatory infiltrate formed of lymphohistiocytic (B)
The onset of the disease happened 1 week after starting antimalarial medication (hydroxychloroquine; 250 mg/day) for SLE treatment, which was thereafter stopped.
Cutaneous examination revealed erythematous-oedematous macules and plaques that were well-defined, spherical, and dusky, with a tendency to grow and evolve into patches. Some lesions were single, while others on the trunk and limbs progressed into many clustered lesions. Scales only adhered to the skin in the centre of the lesions, revealing a pale core. On the mucosa of the hard palate, many petechiae were also observed. Fatigability and myalgia were among the accompanying symptoms. Antibodies against Ro, La, total antinuclear, anti-dsDNA, and rheumatoid factor were all shown to be positive in serological tests.
For pathological evaluation and direct immunofluorescence microscopy, a skin biopsy was obtained from the trunk. The skin biopsy for histopathology was preserved for 48 h at room temperature in a buffered 10% formalin solution. The tissue was then fixed in paraffin, cut into 4-mm slices, and stained with hematoxylin and eosin on a glass microscope slide. A light microscope was used to examine the slides at magnifications of 40, 100, and 200. Basket weave hyperkeratosis, acanthosis of the epidermis with exocytosis, vacuolization and foci of necrotic keratinocytes, and a moderate inflammatory perivascular lymphohistiocytic infiltration were seen histopathologically (Figure 1 B).
The skin biopsy was promptly transferred in cold physiological saline for direct immunofluorescence screening. 4-mm thick cryotome sections were preserved thick cryotome sections were preserved in acetone for 15 min at room temperature. IgA, IgG, IgM, C1q, C3, Fibrinogen were among the antibodies tested. Antibodies that were fluorescently tagged were applied to sections and incubated at room temperature for 30 min. Dako (Agilent Technologies, Inc.) mounting media was used. The locations of attachment of the labelled antibodies on the skin were then identified using a Nikon Eclipse E600 fluorescent microscope at magnifications of 40, 100, and 200. In the basement membrane, direct immunofluorescence revealed complement component 3 and immunoglobulin M.
Rowell syndrome (RS) was diagnosed based on the patient’s history, clinical signs (erythema multiforme-like lesions), antibody detection, histological investigations, and direct immunofluorescence findings. The patient’s skin lesions were completely cured after 1 week with treatment by systemic corticosteroids (methylprednisolone IV; 48 mg/daily) and intravenous immunoglobulin 2 gm/Kg divided over 3 days.
Neville Rowell et al. first described Rowell syndrome (RS) in 1963 as a link between systemic lupus erythematosus (SLE) and erythema multiforme (EM) [1]. A speckled antinuclear antibody (ANA) pattern, anti-Ro/SSA or anti-La/SSB antibody, and positive rheumatoid factor (RF) are all distinguishing immunologic characteristics [1]. Less than 100 cases have been reported since its inception [2, 3]. Women have been observed to have a higher incidence than men, with an 8 : 1 ratio of women to men.
Erythema multiforme is an immune-mediated skin disorder defined by the formation of characteristic target-like lesions on the skin, which are sometimes accompanied by erosions or bullae involving the oral, vaginal, and/or ocular mucosae [4]. Infections, such as herpes simplex virus or Mycoplasma pneumoniae, pharmaceuticals, such as nonsteroidal anti-inflammatory drugs (NSAIDs), antiepileptics, and antibiotics, malignancy, and autoimmune illnesses are also well-known causes of EM [4]. Classical EM is caused by triggers such as infectious agents or medicines, and it is not linked to any specific serological antibodies or chilblains. The presence of serological criteria and a rare overlap of EM and SLE can aid in the diagnosis of RS [1].
RS is an uncommon condition that has been linked to all kinds of SLE. RS was also cited by Beck and Anderson, who described its unique and distinct characteristics [5]. The first identified distinctive characteristics included positive tests for the rheumatoid factor, speckled pattern antinuclear antibody, and precipitating antibodies to saline extract of human tissue (anti-SjT), currently known as La/SSB and Ro/SSA antibodies [6]. Painful, pruritic, annular, erythematous, and red to violet plaques with blisters characterize erythema multiforme-like lesions. The lesions are mostly found on the arms and legs, with the face, neck, chest, and mouth appearing less commonly. They can last from a few days to more than a month [1]. The histopathologic findings of RS are also debatable. Necrotic keratinocytes are observed in 54% of subacute cutaneous lupus erythematosus (SCLE) lesions indicating that they are not exclusive to RS [6].
The direct immunofluorescence of RS and EM reveals similar results in the dermal capillaries, such as deposits of C3 and immunoglobulin M (IgM) similar to SLE. In RS, autoimmune diseases, genetics, hormones, and the environment all play a role. There are no particular autoantibodies linked to Rowell syndrome [6, 7].
Since its original description in 1963, there has been a debate on whether RS is a distinct entity or a side effect of cutaneous lupus erythematosus [1]. Since it was first described, there have been approximately 95 cases reported in the literature [3]. We present a summary of many diagnostic criteria proposed during the last five decades (Table 1). Torchia et al.’s diagnostic criteria appear to be the most precise [8].
Table 1
Diagnostic criteria for Rowell syndrome suggested by various authors
| Author’s criteria | Diagnostic features |
|---|---|
| Zeitouni et al. [1] | 3 major + 1 minor criteria |
| Major criteria: | |
| SLE, DLE, or SCLE | |
| EM-like lesions (with/without involvement of mucous membranes) | |
| Speckled pattern ANA | |
| Minor criteria: | |
| Chilblains | |
| Anti-Ro/SSA or anti-La/SSB | |
| Positive rheumatoid factor | |
| Rowell et al. [5] | All features required |
| LE | |
| EM-like lesions (with the absence of any known precipitating factors) | |
| Speckled pattern of ANA | |
| Anti-SJT antibody (anti-La/SSB) | |
| Positive rheumatoid factor | |
| Lee et al. [10] | All features required |
| LE | |
| EM-like lesions (with the absence of any known precipitating factors) | |
| Chilblains | |
| Speckled pattern ANA | |
| Anti-La/SSB antibody | |
| Positive rheumatoid factor | |
| Torchia et al. [8] | Presence of all 4 major + 1 minor criteria |
| Major criteria: | |
| Presence of DLE, SLE and/or chilblain | |
| Presence of EM-like lesions (typical or atypical targets) | |
| At least one positivity among speckled ANA, anti-Ro/SSA, and anti-La/SSB antibodies | |
| Negative DIF on lesional EM-like lesions | |
| Minor criteria: | |
| Absence of infectious or pharmacologic triggers | |
| Absence of typical EM location (acral and mucosal) | |
| Presence of at least one additional ARA criterion for diagnosis of SLE besides discoid rash and ANA and excluding photosensitivity, malar rash, and oral ulcers |
Treatment response varies, and recurrences are common [6]. The outcomes of several RS treatments have been mixed [6, 8–10]. The use of corticosteroids, IV immunoglobulin, hydroxychloroquine, chloroquine, azathioprine, dapsone, and thalidomide for the treatment of RS has been described [10]. Our patient had excellent results with a combination of methylprednisolone and IV immunoglobulin.
Rituximab is an anti-CD20 monoclonal antibody that is approved for the treatment of non-Hodgkin lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis, but it is also used for a variety of other disorders off label [11]. Its usefulness in treating autoimmune skin illnesses such as pemphigus vulgaris, pemphigus foliaceus, vasculitis, SLE, and SCLE has been demonstrated in studies [12]. Currently, seventeen cases of cutaneous SLE and 8 cases of refractory SCLE treated with rituximab have been reported [12, 13]. Of the eight refractory SCLE cases, seven showed effective results. The dosing regimen varied from 1 g every 2 weeks to 375 mg/m2 weekly for 4 weeks [12]. Maintenance doses varied from every 2 months to 1 year. Although rituximab has shown effective results in treatment of SCLE, it has not been effective for chronic cutaneous lupus erythematosus. Rituximab therapy has been reported in treatment of 5 cases of erythema multiforme major [14, 15].
RS has an excellent prognosis, with most people reporting complete remission after a year. We did not find recurrence of RS for our case after 18 months’ follow-up.
Patients with LE and lesions that resemble EM should be evaluated for RS. Serological and pathological investigations with clinical correlation should be done to reach the final diagnosis of RS.








