Purpose
Treatment of oral or oropharyngeal tumors requires specific knowledge, especially for relapses or second tumors after first local treatment because of anatomical complexity of this region, with frequently associated comorbidities and limitations due to prior treatments. A treatment at the time of a relapse is particularly important because it regulates subsequent life expectancy, with a very low overall survival (OS) in the absence of local control [1]. Surgery is the main treatment option [2] that should always be primarily considered, but often requires adjuvant radiotherapy [3, 4]. Radiotherapy with or without chemotherapy is the only curative treatment option for inoperable lesions, with chemotherapy alone being mostly used as palliative care. Specific treatment schemes that have been developed [5, 6], offer an acceptable compromise between local control and toxicity.
Compared to external beam radiotherapy (EBRT), brachytherapy (BT) allows high doses to be delivered to a well-defined tumor volume while sparing healthy tissue volumes, thus avoiding problems of inter- and intra- fraction motions. BT is therefore a local and potentially curative treatment for tumors of limited extent [7, 8]. However, there is no consensus on its indication in recurrence or new tumors in previously irradiated areas.
Therefore, the aim of this study was to evaluate tumor control and feasibility of BT with or without EBRT for primary or recurrent oral or oropharyngeal tumors in previously irradiated areas.
Material and methods
Patients’ characteristics
The study group consisted of patients treated in our center between 1996 and 2016 for a new pathologically confirmed squamous cell carcinoma (SCC) in a previously irradiated area by salvage BT, either exclusively or in combination with EBRT, and surgery and/or chemotherapy. Only oral cavity or oropharyngeal mucosal sites were considered, and patients with metastases, contraindicated anesthesia, or those with World Health Organization (WHO) performance status of 2 or worse were excluded. p16 status was not routinely performed at that time and was not available for these patients.
Salvage treatment and brachytherapy modalities
Salvage treatment modalities were discussed in a specialized multidisciplinary committee, including head and neck surgeons and medical and radiation oncologists. BT feasibility was assessed clinically by a radiation oncologist experienced in BT. Whenever possible, surgery was firstly performed, followed by adjuvant radiotherapy, including BT alone or in association with EBRT. In this case, EBRT field always involved the tumor site treated with BT. Chemotherapy was considered as neoadjuvant or concomitant treatment.
Implantations were performed under general anesthesia, and a feeding tube was inserted at the end of procedure.
Until November 2014, a low-dose-rate (LDR) BT technique with iridium-192 (192Ir) wires was applied. An afterloading technique with several plastic tubes loops was used, as described by Pernot et al. [9]. The number of wires was defined per-operatively by the radiation oncologist considering target volume and patient’s anatomy, to ensure a proper coverage of clinical target volume. To limit the risk of severe toxicity, the activity of the source was limited to 0.8 mCi/cm.
Patients treated after November 2014 received a pulsed-dose-rate (PDR) BT. The implantation technique had to be modified for the mobile and base of tongue locations because the PDR source was not able to pass through the edge of plastic loops. The loops had to be replaced by two straight plastic tubes positioned with their tips 2-3 mm above the edge of the tongue, using a plastic plate placed on its surface.
Dosimetry was performed on two orthogonal radiography with a 3D reconstruction for LDR, and on a post-operative CT scans with catheter reconstruction and CTV delineation for PDR. LDR treatment time and PDR dwell time were calculated according to Paris system prescription rules. XIO software (CMS) was used for LDR, and Oncentra® (Nucletron V.B., Veenendaal, The Netherlands) for PDR. A graphic and manual optimizations were performed for PDR technique, and dose distribution was optimized to avoid an underdosage at the surface of the tongue. Moreover, PDR technique ensured that each patient received a homogeneous dose-rate of 50 cGy/h (one pulse per hour, 24 hours a day).
Data collection
The patients’ information, survival, tolerance, and toxicity data were obtained from their medical charts and completed by national death register. Adverse events were graded using common toxicity criteria (CTC), version 2.0 [10].
Statistical analysis
All statistical analyses were performed using a software R, version 3.5.1, supported by R Foundation. Results were considered statistically significant at p < 0.05. Continuous variables were summarized by their median, range, and categorical variables using simple counts and proportions. Characteristics of each patient were compared using Mann-Whitney-Wilcoxon test for continuous variables and Fisher exact test for categorical variables.
The primary outcome measure was OS, which was estimated using Kaplan-Meier analysis and compared between patients with log-rank test for variables, including patient’s age, external radiotherapy, chemotherapy, and tumor’s stage at the time of recording. Subsequently, a multivariate Cox proportional hazards model was built to determine the effect of total dose received on patient survival, and adjusted with the age and stage of tumor. As secondary outcomes, local control, disease-free survival, and toxicity were evaluated.
Results
Twenty-eight patients fulfilled the inclusion criteria. Three patients were excluded from the analysis because of missing data (two were lost to follow-up after BT, and in one patient, first treatment data were missing). Characteristics of the 25 patients included are listed in Table 1.
Table 1
Patients’ and treatments’ characteristics (n = 25)
Previous treatment
All included patients were previously treated with EBRT, and four patients also received BT. Radiotherapy was preceded by surgery in 18 patients, and enhanced with chemotherapy for 12 patients. All, except one patient in a partial remission (who was considered as a progressive disease after his first course of treatment), were considered in complete remission at the end of the first course of treatment (Table 1).
Salvage treatment
The patients were mainly men, with a second oral or oropharyngeal tumors, mostly with lesions of the tongue (12 at the base of the tongue and 6 in the mobile tongue). The median interval between initial treatment and salvage BT was 59 months (range, 10-214 months). The tumor was considered as a recurrence in four patients and as a second primary in 21 cases. Tumors’ locations and stages are listed in Table 1.
Three of the 25 patients contraindicated for surgery. Procedures consisted of transoral surgery (15 cases), bucopharyngectomy (2 cases), laryngectomy in case of tumor vallecular extension (4 cases), and in one patient it was unknown. Transoral robotic surgery (TORS) was not used. R1, R2, and Rx resections were in 20, 1, and 1 cases, respectively. Cervical neck dissection was performed in 8 patients (positive in 2 patients). Surgery was followed by adjuvant radiotherapy, with BT alone (14 patients) or in association with EBRT (8 patients). The median time between surgery and radiation therapy was 57 days. In 3 non-operated patients, two received BT + EBRT and one BT alone. Four patients received chemotherapy as a part of salvage treatment (3 neoadjuvant and 1 combined with EBRT). In combined treatments, BT was performed after EBRT for all but one patient, with a median delay of 16 days. Eighteen patients received LDR-BT with 2 to 5 192Ir wires, and the remaining seven obtained PDR-BT with 4 to 8 catheters, as shown in Figures 1 and 2. The median BT dose was 50 Gy (range, 40-64 Gy) and 25 Gy (range, 15-48 Gy) for BT alone and in association with EBRT, respectively. The median total radiotherapy dose received by the patients was 57 Gy (range, 40-70 Gy).
Fig. 1
Base of tongue implant imaging and dosimetry. A 63-year-old woman treated adjuvantly for a second squamous cell carcinoma (SCC), T2 NO, located on the base of tongue, resected with pathological margins. These areas received 50 Gy, 5 years before another SCC, treated by surgery and adjuvant EBRT. BT was delivered with 8 PDR vectors. Red isodose correspond to 55 Gy, green isodose to 30 Gy, and blue isodose to 20 Gy
Overall survival
With a median follow-up of 95 months (range, 18-224 months), the median OS after salvage treatment was 16 months (range, 5-173 months), and 2- and 5-year OS rates were 50% and 26%, respectively. 19 patients died during follow-up at the time of analysis, including 12 due to cancer’s recurrences, 4 from a second cancer (posterior pharyngeal wall tumor in 2, lung cancer in 1, and esophageal cancer in 1 case), and 3 from intercurrent causes. There were no toxicity-related deaths. The only patient considered as having a progressive disease after the first treatment died 96 months after surgery and adjuvant BT.
In the univariate analysis, tumor stage (T1) at the time of salvage treatment was significantly associated with a better median OS: 27 months vs. 14.5 months (p = 0.0467) compared to patients presenting at least T2 tumors (Figure 3). No significant difference was observed according to the total dose level, with a median OS of 16 months vs. 15.5 months for doses under and over 60 Gy, respectively (p = 0.75). OS did not vary significantly with body mass index (BMI), type of tumor (recurrence vs. second tumor), delay between the first and second radiotherapy course, surgery status, or additional chemotherapy.
Fig. 3
Kaplan-Meier overall survival according to total dose (< 60 Gy vs. > 60 Gy) and tumor stage (T1 vs. T2 or more)
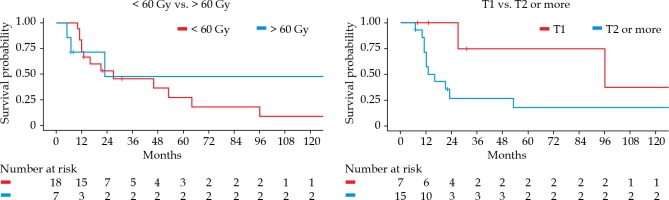
In the multivariate analysis, patients with T1 lesions showed an improved survival compared to patients with more advanced tumors (HR = 6.25, 95% CI: 1.18-33.1%, p = 0.031). Patients treated with a salvage total dose higher than 60 Gy showed a tendency towards better survival (HR = 0.20, 95% CI: 0.03-1.16%, p = 0.072) (Table 2). No difference was found according to age.
Local control
Five local recurrences followed after BT, resulting in 75% of local control in 24 months. All recurrences occurred within 8 months of treatment (range, 2-7 months). Four of these five recurrences were in patients who received a total (EBRT + BT) salvage dose less than 60 Gy (p = 0.396). One of these local recurrences was treated by surgery alone, one by surgery and adjuvant EBRT and chemotherapy. Two patients received palliative chemotherapy, and the remaining patient obtained supportive care alone. All of these patients died within a median survival time from a local relapse of 4 months (range, 3-20 months).
Disease-free survival
The median disease-free survival was 11 months. Six patients experienced a nodal recurrence exclusively, and one patient suffered from a metastatic recurrence (mediastinal recurrence treated by radiochemotherapy) and died 62 months after this new recurrence.
Acute and late severe toxicity
There were four grade 3 acute toxicities, including three cases of soft-tissue necrosis (mucosal necrosis, base of the tongue necrosis associated with a submental fistula and a hyoid bone necrosis). They all occurred in overlap areas, which received a cumulative dose in the range of 107-132 Gy. However, these doses in overlap areas were not significantly higher than in other patients, who did not experience severe acute toxicities. Local symptomatic treatments were successful for all these toxicities, except one, which needed a surgery to cover the defect. There was no grade 3 late toxicity or higher.
Discussion
Patients with a local recurrence or those who experienced a second primary tumor in previously irradiated head and neck areas have a poor prognosis, but some selected patients may still benefit from a curative management. Progressively more data suggest that re-irradiation could be a safe and effective option of treatment [5, 6]. In that case, BT remain the best way to rechallenge recurrent head and neck carcinoma alone or associated with surgery. Most of the previously published studies include patients with different tumor location managed with surgery, chemotherapy, or EBRT [11]. Different BT techniques can be used, such as LDR, PDR, and high-dose-rate (HDR). LDR corresponds to the historical way to practice BT for recurrent head and neck tumors. At the same time, PDR and HDR became increasingly used, thanks to their ability to optimize the delivered dose.
For our analysis, we selected patients with oral cavity and oropharyngeal recurrent tumors only. In that context, only five series have been published [12-16] (Table 3). Mazeron et al. [12] evaluated exclusive LDR-BT in a group of 70 patients with non-operable second oropharyngeal tumor in a previously irradiated area. Doses in range of 60-65 Gy (median, 60 Gy) provided local control and OS rates at 5 years of 69% and 14%, respectively, with 27% of acute and late grade 3 toxicity. Peiffert et al. [13] reported similar results with exclusive salvage LDR-BT in 73 non-operable patients with recurrent carcinoma. Salvage BT permitted to deliver doses ranging from 50 to 75 Gy (median, 60.3 Gy,) with a high local control rate of 78% at 4 years and a 5-year OS rate of 30%. No grade 3 toxicity was reported. In a more recent retrospective analysis, Strnad et al. [14] evaluated outcomes of 51 patients, who developed new oral or oropharyngeal tumors, treated with salvage PDR-BT alone (n = 40) or in combination with EBRT (n = 11) with or without concomitant chemotherapy (n = 35). OS rate at 5 years was 26%, with 10% and 18% of acute and late grade 3 toxicity, respectively. Interestingly, in this study, the addition of chemotherapy, concomitant to BT, resulted in a significant 5-year local control improvement from 38.5% to 78.9%. Bhalavat et al. [14] reported on 25 patients treated with HDR-BT, with a local control rate of 75% at 2 years and only 2% of grade 3 toxicity. They also found that larger tumor treated volume > 85 cc had a significant better OS contrary to volumes < 85 cc (26 months vs. 12 months). In our study involving majority of post-operative treatments, we obtained similar results, with a median OS of 16 months, a 5-year survival rate of 26%, and a 2-year local control rate of 75%. T1 lesions showed an improved survival compared to patients with more advanced tumors (HR = 6.25, 95% CI: 1.18-33.1%, p = 0.031), confirming the impact of tumor volume. Levendag et al. [16] compared outcomes of 55 patients receiving only EBRT with 16 patients, who received BT alone (n = 11) or in combination with EBRT (n = 7). The median total doses received were different: 46 Gy with EBRT alone and 61 Gy with BT ± EBRT. Local control rates were 29% and 50%, respectively, in favor of an impact of total dose on local control.
Table 3
Comparison of our and literature results on salvage oral and oropharyngeal brachytherapy
| Author, year [ref.] | Number of patients | Tumor site | Tumor stage | Previous RT dose (median) | Surgery | Chemotherapy | RT technic (n) | Total dose (median) | Local control | OS (5 years) | Side effects ≥ grade 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mazeron et al., 1987 [12] | 70 | Oropharynx | T1: 23 T2: 35 T3: 12 | N.A. | No | No | LDR | 60 to 65 Gy (60.0) | 72%/2 y 69%/5 y | 14% | Acute and late: 27% 1 patient – grade 5 |
| Levendag et al., 1991 [16] | 18 | Oral cavity/oropharynx | N.A. | 40 to 70 Gy (60) | Salvage surgery: 5 | Chemotherapy: 3 | LDR (n = 11) LDR + EBRT (n = 7) | 35 to 95 Gy (60.6) | 50%/5 y | 20% | Acute: 36% Late: 28% |
| Peiffert et al., 1994 [13] | 73 | Oropharynx | T1: 45 T2: 20 T3/TX: 8 | N.A. | No | No | LDR | 50 to 75 Gy (60.3) | 78%/4 y | 30% | 0% |
| Strnad et al., 2014 [14] | 51 | Oral cavity/oropharynx | T1: 5 T2: 23 T3: 8 T4: 6 TX: 0 | 60 to 76 Gy (65) | No | Concomitant chemotherapy: 35 | PDR (n = 40) PDR + EBRT (n = 11) | BT dose, 12 to 66.3 Gy (57.0) | 57%/5 y | 26% | Acute: 10% Late: 18% |
| Bhalavat et al., 2018 [15] | 25 | Oral cavity/oropharynx | N.A. | 66 Gy | No | No | HDR (n = 18) HDR + EBRT (n = 7) | 40.5 Gy 27.0 Gy | 84%/1 y 75%/2 y | Na | Late: 2% |
| The present study | 25 | Oral cavity/oropharynx | T1: 7 T2: 11 T3: 3 T4: 1 TX: 3 | 50 to 72 Gy (65) | Salvage surgery: 22 | Chemotherapy: 4 | LDR/PDR (n = 15) LDR/PDR + EBRT (n = 10) | 40 to 70 Gy (57.0) | 75%/2 y | 26% | Acute: 16% Late: 0% |
Indeed, the dose has been described as an important factor for tumor control in the literature [17-19]. Salama et al. [17] evaluated different salvage EBRT protocols in a group of 115 patients, of which 48 underwent a resection. These authors found that patients, who received doses greater than 58 Gy had a 3-year survival rate of 30% compared with only 6% for those, who received lower doses (p < 0.001). Bots et al. [18] observed 137 re-irradiated patients, and demonstrated that patients treated with IMRT had a tendency towards better local control than patients treated with conventional technique (49% vs. 36%, p = 0.07). This may be related to median re-irradiation dose, which was higher for patients treated with IMRT (60 Gy vs. 56 Gy, p < 0.05). In the same way, Choe et al. [19] analyzed data of 166 patients of phase I and II protocols. They found a significant benefit in OS while providing at least 60 Gy with EBRT to a recurrent tumor (HR = 0.35, 95% CI: 0.23-0.53%, p < 0.0001) vs. doses under 60 Gy. In our series, even if the total dose delivered by BT and EBRT did not show any significant impact, it seems to be an important therapeutic factor. Actually, in patients treated with doses higher than 60 Gy compared to those who received doses lower than 60 Gy, the risk of death was reduced almost significantly by 80% (HR = 0.20, 95% CI: 0.03-1.16%, p = 0.072). This is reflected by the fact that among the five patients in our study, who suffered a local recurrence (all of them recurred within 7 months following salvage BT), only one initially received a dose greater than 60 Gy (p = 0.396). In terms of toxicity, all grade 3 toxic acute events (4/25) were managed with a symptomatic treatment, except one that needed a surgical intervention. We did not notice any chronic grade 3 toxic event. Only 2 patients in the entire cohort benefited from a flap reconstruction (1 regional and 1 local). This type of reconstruction could potentially have a better re-irradiation tolerance, since the flap was not previously irradiated [3]. With respect to the biases due to non-homogeneous populations and retrospective nature of this study, BT could allow to deliver higher dose to clinical target volume, thereby improving chances of obtaining local control.
Regarding surgery, Narayana et al. [20] reported on 30 patients, including 18 treated with surgical resection and HDR-BT, and 12 treated with HDR-BT ± EBRT, where operated patients benefited from an improved local control at 2 years: 88% vs. 40%, respectively. Similar results were obtained by Hedge et al. [21], who evaluated 30 patients with different head and neck recurrent carcinomas, and presented 73% local control at 2 years and an OS trend in favor of operated patients (p = 0.069). Curiously, in the present study, all the three patients, who presented surgical contraindications were free of any local recurrence during follow-up.
Surgery remains the first therapeutic option in recurrent head and neck carcinoma, but it is frequently associated with necessary adjuvant radiotherapy [3]. There is more data exploring treatment of different recurrent head and neck carcinoma sites [11]. Nodal cervical recurrence is the exact indication of BT, which was described by Anderson, who reported on 51 patients showing 52% of disease-free survival at 2 years after neck dissection with intra-operative PDR-BT [22]. Just as these results appear satisfying, we believe that patients with cervical node recurrences present worse survival outcomes than mucosal recurrent patients, and need to be considered separately. This was confirmed by Breen et al. [23], who analyzed 69 patients treated with LDR. With an overall local control of 38% at 3 years, patients who received BT for a neck disease had significantly worse local control than those, who received BT for a mucosal disease (HR = 2.14, 95% CI: 1.00-4.56%, p = 0.05).
New radiotherapy techniques, such as stereotactic body radiotherapy (SBRT), are still under investigation. Many studies have evaluated their outcomes for head and neck tumor recurrences, including heterogeneous tumors and tumors locations, but none demonstrated the precise validity of SBRT for oral or oropharyngeal SCC recurrences [25, 26]. Immunotherapy or targeted therapies (EGFR inhibitors) demonstrated a potential benefit in patients presenting a recurrent head and neck diseases, but still with a limited median survival, even for selected patients [1, 26, 27]. In that setting, EGFR inhibitors combination were explored by Ritter et al. [28], who investigated 18 patients and compared them to 18 patients, who did not receive concomitant systemic treatment. The results seemed encouraging, showing a beneficial trend towards cetuximab-paclitaxel group, with a disease-free survival and OS of 8.7 and 14.8 months vs. 3.9 and 6.1 months. Moreover, no toxicity increase was observed with this association. To our knowledge, there is no study investigating salvage BT in combination with immunotherapy.
In spite of the biases arising from the small and heterogeneous nature of the sample, this is one of not many studies exploring the value of salvage BT in patients with oropharyngeal or oral cavity tumors in pre-irradiated areas.