Purpose
Interstitial brachytherapy (iBT) has been established as a viable alternative to other loco-regional thermal ablative techniques, such as radiofrequency ablation (RFA) in patients with primary and secondary liver malignancies. It may be applied in cases, where liver lesions are near critical structures or > 3 cm in size. In breast cancer, safety and efficacy of iBT have been established in several studies, showing a high-rate of local tumor control and low complication rates [1, 2]. About 50% of patients with metastatic breast cancer develop liver metastases [3, 4]. Overall survival in this patient’s group is low [5]. Surgical resection or local ablation methods can be offered to a sub-group of patients with metastatic disease limited to the liver [4, 6]. Locally ablative treatments of oligo-metastatic breast cancer may improve survival, and may be applied with a curative intent in selected cases [7-9].
To select proper patients for locally ablative therapies, finding factors that influence prognosis is essential. There has been increasing evidence that sarcopenia, as defined by loss of muscle mass and strength, plays an important role in clinical outcomes in patients with various diseases. Sarcopenia can severely affect cancer patients, as negative protein balance, reduced food intake, and decreased physical activity can lead to progressive muscle loss [10, 11]. Sarcopenia has also been identified as a poor prognostic marker for numerous oncologic diseases. For malignancies, such as non-metastatic colorectal cancer (CRC), gastric, esophageal, pancreatic cancer, and lymphoma, sarcopenia was associated with worse overall survival [12-16]. In a meta-analysis, patients with sarcopenia showed higher dose-limiting toxicity (DLT) [17].
For breast cancer, sarcopenia has been shown to correlate with an increased mortality and treatment toxicity in non-metastatic and metastatic breast cancers [18-25]; however, the data is rarely compared with other common cancers [26]. The influence of sarcopenia on non-surgical local treatments in breast cancer liver metastases (BCLM) is unknown to date.
The aim of this study was to assess the influence of sarcopenia on overall survival of patients undergoing iBT for BCLM. We applied psoas muscle index (PMI) for measurements of sarcopenia. Additionally, psoas muscle density and skeletal muscle gauge (SMG) were calculated.
Material and methods
Study design
From our database, we retrospectively identified 97 patients with breast cancer liver metastases, who received iBT at our institution from 2006-2017. All 97 patients were seen at our department for follow-up visits every 3-6 months after therapy. Patients were followed up until 2020. The study was approved by a local ethics committee (145/21).
Inclusion criteria were:
confirmed metastatic breast cancer with liver metastases;
available CT scan including the psoas muscle on L3 level before treatment,
available clinical data regarding overall survival (OS).
Exclusion criteria were:
Imaging analysis
All CT scans were obtained from a multidetector CT scanner (Canon Aquilion Prime, Otawara, Japan). Patients were positioned in a supine position. CT protocol was as follows: acquisition slice thickness of 1 mm with 5 mm reconstructions, tube voltage of 120 kV, automatic tube current modulation, pitch factor of 1.2, and collimation 1 mm.
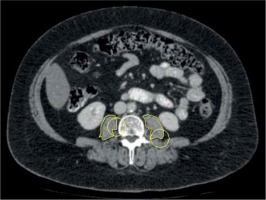
We referred to the last available pre-treatment CT scan within three months prior to iBT. All CT measurements were performed by two experienced radiologists (MT and AS) with 3 and 16 years of experience, respectively. Both were blinded to the clinical course of patients. Measurements were performed on axial images at the mid-L3 level in soft tissue window (Window 45 to 250 HU) on a dedicated workstation (Infinitt PACS, version 3.0, Infinitt Healthcare, Korea). A line was drawn manually along contours of the psoas muscles on both sides, and bilateral areas as calculated by the software were added to obtain PMA (Figure 1). Muscle density was determined by the software for each psoas area and the mean was calculated for both sides. PMI was calculated by dividing PMA by patient’s body height. SMG was determined multiplying PMI with mean muscle density, as previously reported [27]. The parameter integrated both PMI and muscle density, and has been shown to be associated with outcomes in breast cancer patients [23, 27]. SMG units are cm2 × HU/m2, but were given as arbitrary units (AU) for simplicity. Sarcopenia was defined as PMI < 5.45 cm2/m2 for males, and < 3.85 cm2/m2 for females [10].
Statistical analysis
We used SPSS version 26 for statistical analysis. For continuous variables, mean and standard deviation as well as median and interquartile range (IQR) were calculated. To assess the impact of psoas muscle composition on survival, we used a univariate Cox regression analysis. For factors with a significance value of p < 0.1, adjusted prognostic ability to predict overall survival was further assessed using a multivariate Cox regression, with forward selection. Odds ratios were presented together with 95% confidence intervals (95% CI).
Results
Altogether, 60 patients met the inclusion criteria and were included in our analysis. The median age was 56 years. 27 patients (45%) had an skeletal muscle index (SMI) below the cut-off value, and were considered sarcopenic. The median time between CT scan and therapy was 1 day (range, 0-95 days). By the time of treatment, the primary breast tumor had been resected in 57 patients; 39 patients had received radiotherapy, 58 patients had received chemotherapy prior to iBT, and 31 patients had undergone systemic therapy with trastuzumab. Selective internal radiation therapy (SIRT) of the liver had been performed in nine patients. The median tumor size was 2.9 cm. No major intervention-associated complications were observed. Baseline patients’ characteristics are summarized in Table 1.
Table 1
Patients’ baseline characteristics. For body composition, sarcopenia was defined as PMI of < 5.45 cm2/m2 for males and < 3.85 cm2/m2 for females
Overall survival
The median overall survival was 27 months (SD = 4.0 months) (Figure 2). In a univariate Cox regression, neither PMA (HR = 0.956, 95% CI: 0.855-1.068, p = 0.423), average density (HR = 1.028, 95% CI: 0.985-1.072, p = 0.207), nor SMG (HR = 1.002, 95% CI: 0.998-1.006, p = 0.440) were associated with overall survival. There was no influence of sarcopenia on OS (HR = 0.975, 95% CI: 0.532-1.787, p = 0.934). Also there was no proportional influence of PMI on OS (HR = 0.951, 95% CI: 0.701-1.290, p = 0.746). Regression results are presented in Table 2.
Fig. 2
Kaplan-Meier overall survival curves for patients with sarcopenia and without sarcopenia as measured by PMI (log-rank test 0.933)
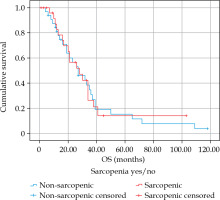
Table 2
Results of regression analysis for overall survival
Discussion
Our study assessed the impact of sarcopenia on overall survival of patients undergoing iBT for breast cancer liver metastases. To the best of our knowledge, this is the first study evaluating the association of sarcopenia with patients’ outcomes in non-surgical local treatments in metastatic breast cancers. We did not find an association between sarcopenia, psoas muscle density, or SMG with overall survival in our cohort.
Breast cancer is among the leading causes of cancer-related death in women around the world [25, 28]. Liver metastases are common in breast cancer patients, and significantly worsen survival if left untreated. While advanced stage breast cancer patients usually receive systemic therapy, studies have shown that a carefully selected group of breast cancer patients with liver metastases profit from loco-regional treatments, when compared to systemic therapy alone [3, 29, 30]. Non-surgical local treatments, such as radiofrequency ablation (RFA), microwave ablation (MWA), and iBT are minimally invasive procedures and viable alternatives to resection, and are the first choice for unresectable liver metastases.
Outcome’s prediction is an important parameter in interventional medicine, and a key step to individualized medicine. Sarcopenia is a complex syndrome and common in oncologic patients. Its’ etiology is diverse, including inflammation, disuse, and low nutritional intake [25]. Multiple studies have shown the influence of sarcopenia on treatment outcomes in cancer patients [31-33]. Sarcopenia has been identified as a poor prognostic marker in numerous cancers, including gastrointestinal tumors, lung cancer, head and neck cancer, and ovarian cancer [31-41]. It has been associated with increased post-operative complications, improved toxicity of systemic therapies, and decreased overall survival [17, 42-44]. Several meta-analyses among gynecological cancer patients showed an influence of sarcopenia on OS and progression-free survival (PFS). However, the definition of sarcopenia across the studies was not standardized [45-47].The relationship for some tumor entities is still undetermined [48].
It has been shown that sarcopenia as measured on CT scans is an important prognostic factor in non-metastatic breast cancer patients [18, 26, 49]. A recent meta-analysis showed that mortality in cancer patients increased with a decrease in lean mass [50]. In a meta-analysis including 81 studies, low lean mass was associated with an decreased OS across different definitions, including the international consensus of cancer cachexia and different cut-off points for skeletal muscle mass [51]. Huang et al. identified low PMI at the L4 level to be associated with overall survival and distant metastases-free survival before surgery [52]. Low skeletal muscle mass is therefore a relevant patient-related condition that warrants early detection for optimal prevention, prognostication, and management.
In metastatic breast cancer, the rate of sarcopenic patients has been reported to be as high as 45% in a recent meta-analysis [25]. An association with mortality was found for non-metastatic breast cancer, but not for metastatic sub-group. In other studies, sarcopenic patients with non-metastatic breast cancer had lower OS [18, 53]. Shachar et al. found an association between SMG and higher treatment-related toxicity, hospitalization, and time to treatment failure [23]. Aleixo et al. reported higher mortality and chemotherapy toxicity in breast cancer patients with low SMI [54]. In contrast, Rier et al. observed that low muscle density was associated with lower OS in patients with metastatic breast cancer, while SMI was not [21]. In a sub-analysis in a large meta-analysis by Au et al., overall mortality in breast cancer patients was not associated with sarcopenia. However, the analysis included only three studies. Measurement of lean mass was heterogeneous, with both bio-impedance analysis and computed tomography included. Sub-analyses for patients with metastases were not performed [50].
There are yet no studies investigating the influence of sarcopenia for breast cancer patients undergoing loco-regional therapies. For RFA, several studies showed that low pre-treatment PMI and SMI were associated with lower OS in HCC patients after RFA [55-57]. Similarly, a negative influence of sarcopenia as measured by PMI and SMI on OS was found in patients undergoing trans-arterial chemo-embolization (TACE) and trans-arterial embolization (TAE) [58, 59].
In contrast to these data, sarcopenia did not affect overall survival after iBT in our cohort. This might suggest that iBT may be a reasonable therapeutic option in sarcopenic BCLM patients undergoing non-surgical local therapy. If sarcopenic patients do not show worse overall survival after iBT, this could be an important parameter in patients’ allocation. Factors that predict outcomes after iBT are still scarce, but no effect of clinical variables on outcomes has been reported. It has been shown that increasing tumor size and applied radiation affect local recurrence rate, while age and clinical variables, such as comorbidities, did not [60-62]. Repeated iBT treatments demonstrated the highest effect on overall survival in a study by Ricke et al. [61].
Our study indicates that screening for sarcopenia is important prior to BCLM therapy. Measuring skeletal muscle mass is a clinical useful tool that may influence treatment decision. Unlike other factors influencing survival, sarcopenia is modifiable. Screening via CT imaging is easy to integrate into clinical routine. Early identification of sarcopenia may induce multimodal interventions and improve patient outcomes. With more data available, it may also be worth considering using sarcopenia as an additional factor to allocate patients to specific treatment arms based on individual assessment. Our data show that sarcopenia is not a limiting factor in BCLM patients undergoing iBT. Additional comparative studies with surgical and other locally ablative procedures will be needed to evaluate whether this translates into an actual survival benefit. The percentage of sarcopenic patients in our cohort corresponds to those found in other studies, yet analyses with higher patient numbers will be needed to confirm our results and individualize therapy lines.
Our study has several limitations that need to be considered. It was a retrospective study at a single institution. Not all patients received a CT scan within 100 days prior to therapy, leading to exclusions and potential bias. We applied PMI as an indicator of sarcopenia; the effect of SMI or other measures of sarcopenia was not evaluated. Nevertheless, to the best of our knowledge, this was the largest study investigating the impact of sarcopenia on survival in patients undergoing locally ablative therapies for BCLM.
In conclusion, our retrospective analysis reveals that sarcopenic patients do not show decreased overall survival when undergoing iBT for BCLM. Neither investigated body composition parameter showed influence on survival time. Our results suggest that iBT may be considered as a treatment option for sarcopenic patients with BCLM. Matched cohort studies comparing iBT with other local treatment strategies are warranted.