Introduction
Currently, endometrial cancer is the most common gynecological malignancy and the fourth most common malignant neoplasm in the female population. It affects most commonly peri- and postmenopausal women. It is markedly age dependent, its frequency being higher in women over 60 years of age and significantly lower in women under 40 [1, 2]. Early menarche, late menopause, advanced age, obesity, diabetes, menstrual irregularities, polycystic ovary syndrome, nulliparity, hormone replacement therapy, and estrogen producing tumors are all well-known factors which contribute to the increased risk of developing endometrial cancer [3].
Two different types of endometrial cancer can be distinguished – type I and type II. The first type is known as estrogen dependent and predominantly affects peri- and postmenopausal women. It is well differentiated and has a better prognosis. Type II is estrogen independent and affects postmenopausal women, has a more aggressive course and a poor prognosis compared to type I [4, 5].
Fibronectin (FN) is a protein with high molecular weight, which is part of the extracellular matrix. It was first described in 1948. In 1975 Ruoslahti and Vaheri also discovered a large multi-domain glycoprotein on the surface of fibroblasts, which was named “surface fibronectin” [6]. It is essential for cellular growth, migration and differentiation, intercellular adhesion, and cytoskeletal organization. Altered FN expression and degradation are associated with a number of diseases, one of them being cancer. There are lower levels of FN in patients with pemphigus than healthy patients [7], which can be used as marker of disease severity in patients with COVID-19 [8], FN was elevated in patients with hepatocellular cancer, with levels falling in treated patients [9].
There are two different types of FN: soluble (plasma FN) and insoluble (cellular FN). Soluble FN is found in the amniotic fluid, the cerebrospinal fluid, the allantois, the synovial fluid, and the cellular basal membrane. Cellular FN is found in fibroblasts, hepatocytes and endothelial cells.
The two main types of FN share similar physical and chemical characteristics, but differ in their biological activity and their effect on cells undergoing malignant transformation. Of the two, the plasma FN is the most studied.
Recently, there has been an increased interest in FN levels due to its association with malignant transformation. It has been found that the addition of FN to a tumor cell induces changes in its physiology. The lack of FN in malignant cells has been demonstrated in vitro, which is why its role in metastasis has been discussed. Fibronectin is present in many cell types and is extremely important for vertebrate organisms, which was proven by George et al. in 1993 in a study which found that inactivation the FN gene leads to early embryonal death in mice [10].
Although FN has been studied for more than two decades, this remarkably complex molecule is still a subject of scientific investigation, which led to the discovery of new integrin and heparin binding sites [11, 12] as well as a new form of the molecule [13], which mediates a particular viral infection [14].
Fibronectin usually exists as a dimer, composed of two nearly identical subunits. Each monomer is composed of three types of repeating units: type I, type II, and type III. All three types of FN repeats are found in other molecules, which suggests that it evolves via exon “shuffling” [15].
The objective of the study is to measure the serum levels of FN in patients with endometrial cancer and to compare them with patients with benign pathology and a control group.
Material and methods
Study population
To measure the levels of FN, serum was taken according to pre-standardized criteria from a total 211 patients aged 30–86 years between 19.05.2020 and 05.10.2021 (Table 1). All participants in the study are patients of the First and Second Gynecological Department at the University Hospital “Maichin dom” – Sofia.
Table 1
Patients’ distribution by age
The participants in the study were selected according to the following inclusion criteria:
Women with abnormal uterine bleeding in fertile, pre-/peri- and postmenopausal age;
Asymptomatic women with an incidental ultrasound finding suspicious for endometrial pathology;
Symptomatic patients with ultrasound findings, associated with pathology of the endometrium;
Histologically confirmed cancer of the endometrium (D and C);
Absence of other benign or malignant diseases of genital and extragenital origin;
Age over 30 years.
The following exclusion criteria were applied in the sample selection:
Benign diseases of the myometrium such as uterine fibroids and adenomyosis;
Uterine malignancies other than endometrial cancer;
Benign and malignant tumors of ovarian origin;
History of or currently diagnosed neoplasia of extragenital origin;
Systemic diseases (autoimmune, liver diseases, kidney diseases, etc.).
A total of 211 samples were collected, 29 of which dropped out due to the exclusion criteria. The remaining samples were divided into three groups as follows: 50 samples from patients with histologically verified endometrial cancer, 50 samples from patients with histologically confirmed endometrial polyps and 50 samples from healthy patients.
The control group samples were collected from women without concomitant diseases and without evidence of endometrial and other genital pathology, in accordance with the inclusion and exclusion criteria. As total of 82 samples from healthy patients were collected, and 50 were subjected to analysis in the study. The remaining 32 were not included, due to the limited capacity of the kits and because in some of the patients the absence of endometrial pathology could not be excluded histologically (patients after diagnostic laparoscopy, plastic surgery of the vagina, operative interventions for stress urinary incontinence – Burch, Marshall-Marchetti-Krantz procedures).
The 50 samples from healthy patients in the study were distributed, according to the surgical procedure they had undergone, as follows: hysteroscopy – 24% (12 patients), dilation and curettage – 32% (16 patients) and vaginal hysterectomy – 44% (22 patients).
Before enlisting in the study, every patient gave their consent and filled out a questionnaire for obtaining information about the current diagnosis, height, weight, age, blood pressure, harmful habits, education, place of work, age of menarche, first day of last menstrual period, number of pregnancies, accompanying diseases, previous surgical interventions, family history, and result of histological examination of the endometrium. Each patient in the study filled out an informed consent form prior to any blood tests, declaring their willingness to participate.
All samples were processed and examined in strict compliance with the rules and standards of the Department of Clinical Laboratory and Clinical Pharmacology of University Hospital “Alexandrovska” – Sofia
Methods
Venous blood was withdrawn from all included patients and serum was separated and stored in aliquots at –20°C. The subjects were divided into three groups: women with endometrial cancer; with endometrial polyps; and the control group.
Human FN measured in mg/ml was analyzed via enzyme-linked immunosorbent assay (ELISA).
Histopathological examination was performed at the Department of Pathology at the University Hospital “Maichin dom” – Sofia.
Data analysis
Before selecting the method to compare the values between the three groups, we analyzed the deviation from the normal distribution of the serum FN levels using the D’Agostino-Pearson test. A significant deviation of FN values from the normal distribution was found – FN (K2 = 57.06, p = 0.001). The Kruskal-Wallis test was used and, additionally, in order to evaluate potential deviations, we investigated whether the results would be confirmed via analysis of variance (ANOVA). We established that the results for intergroup differences did not change.
Results
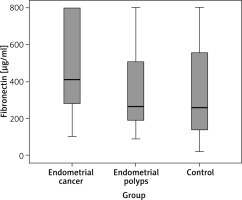
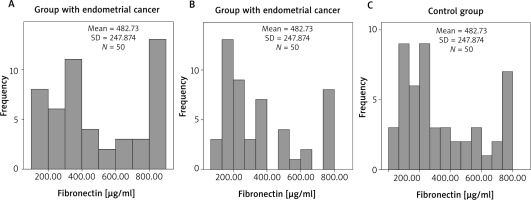
Our results in each of the three groups were graphically presented. We presented the serum FN levels in each group and determined their frequency, mean value and standard deviation. (Fig. 1 A–C). After that we summarized their distribution (Fig. 2). We found that the FN values deviated from the normal distribution, which justifies the use of a nonparametric test in order to make a comparison between the three groups. In addition, in the control as well as in the polyp group the distribution of both indicators was shifted to lower values. The Kruskal-Wallis test was used and it was significant for intergroup differences (10.11, df = 2, p = 0.006), showing that the FN values in the carcinoma group (Fig. 1 A) (mean 482.73, median 409.12 µg/ml) were significantly higher (p = 0.008) compared to the control group (Fig. 1 C) (mean 346.86, median 258.87 µg/ml). However, the levels of FN in the polyp group (Fig. 1 B) (mean 364.21, median 264.25 µg/ml) did not differ significantly from the those of the control group (p = 1.000). The difference in FN levels between the cancer and polyp group nearly reached statistical significance (p = 0.054) (Fig. 2).
Fig. 1
Fibronectin levels in the groups: endometrial cancer (A), endometrial polyps (B), control group (C)
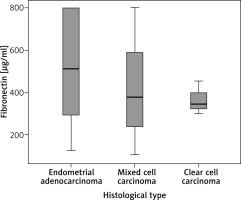
We also analyzed the serum FN levels depending on the histologic type in the endometrial cancer group. The distribution in this group, which consists of samples from 50 patients, is as follows: 28 women (56%) with endometroid adenocarcinoma, 19 women (38%) with mixed cell endometrial carcinoma and 3 women (6%) with clear-cell carcinoma. After analyzing the results from the samples, we found no significant differences among the histological variants (1.81, df = 2, p = 0.613) between endometroid (mean 524.43, median 487.82 µg/ml), mixed cell (mean 416.14, median 377.04 µg/ml) and clear cell (mean 364.84, median 342.58 µg/ml) carcinoma (Fig. 3).
Discussion
Fibronectin has a wide variety of functions – in addition to binding to cell surfaces via integrins, it also binds to a number of biologically important molecules, including heparin, collagen/gelatin and fibrin. These interactions are mediated by several different structural and functional domains, which are defined by proteolytic fragmentation and analysis of recombinant DNA.
Gao et al. suggested that fibronectin 1 (FN1), a member of the FN family, plays a role in a variety of biological processes, which include cellular adhesion, migration and cytoskeletal organization both in the presence as well as in the absence of disease [16].
In a variety of malignant neoplasms, such as carcinoma of the nasopharynx, the esophagus and the ovary, as well as osteosarcoma, FN1 proved to be an important tumor-related gene [17–23]. In regard to ovarian cancer, for example, Bao et al. compared two different cell lines with different migration and invasion abilities and found FN1 to be a potential potent candidate marker for the diagnosis of aggressive ovarian cancer. The same authors suggested that FN1 could play a useful role by providing information on whether ovarian cancer has progressed or metastasized [21].
Similar observations were made by Franke et al. and Kujawa et al. [22, 23].
In their study Bao et al. examined the FN1 expression levels in patients, using next-generation sequencing [21]. They found that FN1 expression was elevated in those diagnosed with ovarian cancer, compared to a healthy control group. In addition, the expression of FN1 in FIGO stage III cancers was significantly elevated compared with stages I and II, suggesting that higher FN1 expression correlates with more advanced disease. These results demonstrated that measuring FN1 levels has the potential to become a useful marker in malignant ovarian disease. [22].
Other authors such as Grammatikakis et al. studied FN plasma levels in patients with gynecological malignancies and in healthy women. They determined that the plasma levels were significantly elevated in the group with malignancies compared to the control group [24].
An important question in recent years is whether FN values can be a reliable marker for gynecological malignancies. In our study a statistically significant difference in FN levels was found in women with malignant endometrial pathology compared to the control group. The results we obtained when comparing FN levels of women with malignant and benign endometrial pathology nearly reached statistical significance.
Given that endometrial carcinoma is the most common gynecological pathology and its frequency is increasing worldwide, the significance of the disease is determined by the search for additional biomarkers with the aim to make an earlier diagnosis and prompt treatment more possible. Although FN has been studied for more than two decades, this molecule is still an object of serious scientific interest due to the fact that its role and influence on tumorigenesis and the spread of malignant processes have been established. Worldwide, few studies have been conducted on serum FN levels in patients with benign and malignant gynecological diseases. It is for this reason that our study was aimed at analyzing FN levels in the most common diseases affecting the endometrium. The majority of the studies examined samples from tissue cultures, whereas in our study we analyzed serum FN levels in women with endometrial pathology and a control group. The observed trend in the data obtained from our prospective study supports the results of other studies and indicates that the serum FN concentration can be used as an additional tumor marker for gynecological malignancies. Our study confirms the need for further studies covering an even larger group of patients to determine whether FN can be a potential diagnostic and prognostic marker for malignant endometrial pathology as well as for other gynecological malignancies.
We acknowledge the fact that a weakness of our study is the small number (50) of analyzed samples of women with endometrial carcinoma, and that of them, those with type I endometrial carcinoma significantly predominated, while those with type II constituted a very small part.
Conclusions
In the study a statistically significant difference was observed in the serum FN levels between the cancer and control groups. We found no difference between the polyp and control groups and between different histological types of endometrial cancer and a borderline statistically significant difference between the cancer and polyp groups. Therefore, the levels of FN1 can be used as an additional tumor marker for endometrial cancer in addition to normal tissue and cannot be used as a marker for differentiation between the different endometrial cancer types.