Sepsis is a syndrome of physiological, pathological, and biochemical abnormalities, with several pathophysiological processes that occur simultaneously [1]. The worldwide incidence of sepsis is increasing [2–6], and therefore the correct understanding of its pathophysiology, diagnosis, and treatment are important. Depending on the definition used, incidence in the United States varies between 300 per 100,000 and 1031 per 100,000 inhabitants, with an annual growth of 13% [4]. Hospital mortality varies between 14.7% and 29% [4]. In some studies, carried out in Latin-America, it is even higher [7–10].
Among the pathophysiological processes, mitochondrial dysfunction, which is caused by a decreased function of certain complexes in the electron transport chain, producing less energy and higher levels of free radicals, can be found. In vivo, reactive oxygen species (ROS) are produced in different pathways, different cells, and in different quantities. In severe pathologies, phagocytes are the main cells that produce ROS as an element of immune defence, and their purpose is the destruction of microorganisms. Under normal circumstances, there is a continuous production of small amounts of ROS in the mitochondria and, more precisely, in the electron transport chain [11]. It is believed that the latter is responsible for 90% of ROS produced in the cell [12, 13]. Immune system and mitochondrial dysfunction are the main sources of ROS. In sepsis, ROS and reactive nitrogen species (RNS) are increased, both in circulation and in tissue [14].
The plasma oxidative burst takes place in the early stages of sepsis and is carried out by neutrophils, macrophages, and endothelial cells. Antioxidants remove ROS from the plasma, which leads to a decrease in the concentration of these molecules [15]. The level of free thiols decreases due to oxidation or nitrosylation [15]. As a result, the level of antioxidant capacity of plasma is reduced [15]. There are several proteins that activate signalling pathways that promote apoptosis and are sensitive to superoxide [16]. Also, hydroxyl radical is a reactive species that oxidizes lipids, proteins, and DNA, which results in genomic instability [17].
On the other hand, another pathophysiological process described in sepsis is apoptosis. Apoptosis is a highly regulated and conserved cell death process during which the cell self-destructs [16]. It is a normal process, which, in multicellular organisms, eliminates undesired or superfluous cells, neutralizing the potential damage caused by cells with defective DNA [16].
Cells in which apoptosis is triggered decrease in size and are condensed and fragmented, releasing membrane-limited apoptotic bodies that are generally absorbed by other cells [18]. In addition, the nucleus is condensed, the DNA is fragmented, and the cellular constituents are not released to the extracellular medium where they could harm the surrounding cells, in contrast with what happens in cell death by necrosis [18]. Apoptosis involves enzymes that cleave proteins at the cysteine amino acid level (caspases) [18]. Most, but not all, of the apoptotic pathways lead to the activation of these enzymes [16].
Different triggers have been proposed as the cause of organ failure: Decreased tissue perfusion and its resulting respiratory failure, mitochondrial dysfunction, the production of ROS, and other mecha-nisms, cell death being the final outcome [19].
In recent years, interest in apoptosis as a mecha-nism of cell death has increased. There are studies that show increased levels of apoptosis at the cellular level in different models of sepsis [20–23], but there is insufficient evidence regarding systemic apoptosis levels in septic patients, or the relationship with mortality in intensive care units (ICU) [24, 25]. While several organs show evidence of inflammation with migration of inflammatory cells, increased interstitial fluid, increased capillary permeability, and epithelial disruption, the degree of cell death is low [26]. The degree of cell death is disproportionately low compared with the degree of clinical severity or the biochemical presentation of organ dysfunction [26, 27]. Also, there is indirect evidence of lymphocyte apoptosis in septic patients with extensive depletion of lymphocytes in the white pulp of the spleen [28].
Both pathophysiological processes (ROS production and apoptosis) and others like inflammation, thrombosis, microabscess formation, and ischaemic changes are causes of the multiple organ dysfunction observed in sepsis, which, if left uncontrolled, leads to patient death [29]. The aim of this study was to investigate the level of serum markers of ROS and apoptosis and to ascertain whether those processes occur simultaneously. There is no literature relating ROS levels and apoptosis in sepsis.
METHODS
Study design
This is a prospective observational trial, carried out in a single centre. The centre is a private tertiary hospital with an intensive care unit with 27 beds, in Buenos Aires, Argentina. Subjects were included between May 2013 and December 2017. The protocol was approved by the local internal review board (number of approval: CRIHB#521). All patients signed an informed consent form.
Inclusion and exclusion criteria
Inclusion criteria for the patients were as follows: aged between 18 and 80 years with a diagnosis of sepsis, according to the Sepsis-2 definition [30]. Inclusion criteria for the healthy volunteers were as follows: over 18 years of age with no actual septic pathology or signs. Exclusion criteria for the septic patients were the following: refusal of informed consent, limitation of therapeutic efforts at the time of inclusion in the study, and pregnancy.
Treatment of sepsis
The diagnosis and treatment of sepsis was decided by ICU physicians. For treatment, the recommendations of the 2013 and 2016 “Surviving Sepsis Campaign” guides were followed [2, 31].
Measurement of serum reactive oxygen species
Upon arrival of the patient, within 12 hours of admission to the ICU, venous blood samples were taken. After that, blood was centrifuged for 5 minutes at 3000 rpm, and serum was frozen at –70°C, as described in the bibliography [32–34]. Using dichlorofluorescein-diacetate (DCFH) (Sigma Aldrich. Catalogue D6665) ROS were measured. 12 µL of serum were incubated for 10 minutes in 1000 µL of TE buffer, and 10 µL of NaOH and 10 µL of DCFH solution were added. Fluorescence was measured after creating an emission spectrum between 500 and 550 nm with each serum sample; the peak of emission at 525 nm was recorded.
Measurement of serum levels of caspase-3
In the same sample described for the measurement of serum ROS, the quantification of caspase-3 levels was carried out using the ELISA method on patients’ serum. The kit was manufactured by MyBioSource® – Human Caspase-3 ELISA KIT Catalogue #MBS040290, batches #02/2016 and #10/2017. In order to do this, the patients’ serum, frozen at –70°C, was used. The levels of caspase-3 are shown in pg mL–1. Samples were measured using an EMP M201® Microplate Reader (China).
Statistical analysis
Normal and non-normal distribution data were determined based on mean, median, and kurtosis. The mean was calculated with standard deviation (SD) to describe quantitative variables in normal distribution data, and with 95% confidence interval (95% CI) in non-normal data. In order to analyse whether there were significant differences in continuous variables between two groups, Student’s t-test was used for variables with normal distribution, and the U Mann-Witney test was used for variables with abnormal distribution. To compare nominal and ordinal qualitative variables, the chi-square test was used. To correlate two variables, the Spearman test was used, depending on whether the distribution of the variables was normal or not. In all cases, a significance level of P < 0.05 was considered. The following statistical programs were used: EPIinfo 7.0, Statistix 7.0 (Informer Technologies, Inc., Atlanta, USA), and Graph Pad Prism 8.0.2. (GraphPad Software, San Diego, USA).
RESULTS
Table 1 shows the comparison of demographic and clinical parameters between patients and healthy volunteers. A total of 124 people were enrolled, 98 of whom were patients with severe sepsis or septic shock diagnoses, and 26 were healthy volunteers. None of the healthy volunteers had a chronic disease that required treatment. The healthy volunteers were younger than the patients. Table 2 describes the severity of disease scores and origin of infection.
TABLE 1
Description of the population enrolled in this study
TABLE 2
Description of the septic patient population. The characteristics of the septic patient population are described. The APACHE II score values were taken with the worst result in the first 24 hours and the SOFA score with the values at admission
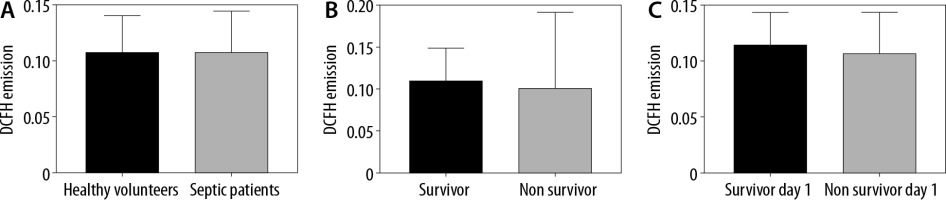
Serum ROS were measured using the DCFH technique. There were no differences in the ROS levels between healthy volunteers and septic patients (Emission = 0.1077 [SD = 0.0324] vs. 0.108 [SD = 0.0364]; P = 0.26) (Figure 1A). Twenty-eight out of the 98 patients died during hospitalization. When analysing only septic patients, there were no differences in the ROS levels of patients who survived and those who died in the ICU (Emission = 0.1103 [SD = 0.0384] vs. 0.1022 [SD = 0.0303]; P = 0.089) (Figure 1B). Finally, 7 patients died during the first day of hospitalization, and there were no differences between those patients and the patients who survived (Emission = 0.1076 [SD =0.0369] vs. 0.1145 [0.029]; P = 0.31) (Figure 1C).
FIGURE 1
Serum reactive oxygen species (ROS) levels. A) ROS levels in healthy volunteers and patients. Dichlorofluorescein-diacetate (DCFH) emission levels are stated at 525 nm as a marker of ROS. There was no significant difference between groups. Student’s test (P = 0.26). B) ROS levels of patients who lived and died in the ICU. DCFH emission levels are stated at 525 nm as a marker. There was no significant difference between groups. Student’s test (P = 0.089). C) ROS levels in septic patients who lived and those who died on the first day of hospitalization. DCFH emission levels are stated at 525 nm as a marker of ROS. There was no significant difference between groups. Student’s test (P = 0.31)
Serum levels of caspase-3 were higher than those of healthy volunteers (31.75 pg mL-1 [95% CI: 25.84–37.65] vs. 7.67 pg mL-1 [95% CI: 1.93–13.4]; P < 0.001) (Figure 2A). When analysing only patients, there were no statistically significant differences between patients who lived and those who died during their hospitalization in the ICU (28.49 pg mL-1 [95% CI: 22.21–34.77] vs. 39.89 pg mL–1 [95% CI: 26.22–53.57]; P = 0.18) (Figure 2B). Finally, there were no significant differences in the levels of caspase-3 between patients who survived and those who died on the first day (29.83 pg mL–1 [95% CI: 23.99–35.67] vs. 56.69 pg mL-1 [95% CI: 21.82–91.56]; P =0.1) (Figure 2C).
FIGURE 2
Serum apoptosis levels. A) Serum levels of Caspase-3 in healthy volunteers and septic patients. There were significant differences between groups when using the Mann Whitney test (P < 0.001). B) Serum levels of Caspase-3 in patients who lived and those who died in the ICU. There were no significant differences between groups. Mann Whitney test (P = 0.18). C) Serum levels of Caspase-3 in patients who lived and those who died during the first day of hospitalization. There were no significant differences between groups. Mann Whitney test (P = 0.1)

The Spearman test was performed to demonstrate any correlation between DCFH emission levels and serum levels of caspase-3 of septic patients and those of healthy volunteers. There was no correlation between the levels of both variables. A scatter plot with levels of both variables was made (Figure 3). In addition, the Spearman test was also performed to demonstrate any correlation between DCFH emission levels and serum levels of caspase-3 only among septic patients. There was no correlation between the levels of both variables. A scatter plot with levels of both variables was constructed (Figure 4).
FIGURE 3
Correlation between reactive oxygen species (ROS) levels and serum levels of caspase-3. ROS were measured using the dichlorofluorescein-diacetate (DCFH) technique and serum levels of caspase-3 were measured in healthy volunteers and controls. There was no correlation between both variables (R = –0.0013, P = 0.98)

FIGURE 4
Correlation between systemic reactive oxygen species (ROS) levels and serum levels of caspase-3 in septic patients. There was no correlation between both variables (R = –0.0732, P = 0.47)

Finally, correlation between serum ROS and serum caspase-3 levels with the severity scores SOFA and APACHE II was also analysed. There was no correlation between the levels of caspase-3 and the SOFA score (R = 0.1823; P = 0.08), nor with the APACHE II score (R = 0.1047; P = 0.32). There was also no correlation between APACHE II levels and ROS levels (R = –0.0562; P = 0.6). In contrast, there was an inverse correlation between SOFA levels and activated ROS levels (R = –0.2146; P = 0.04).
DISCUSSION
In this study, we included 98 septic patients and 26 healthy volunteers. All patients were admitted to the ICU with severe sepsis and septic shock diagnoses, in accordance with the second definition of sepsis, which was valid at the start of the study [30].However, considering the Sepsis-3 consensus [1], the same group of patients would have been included. This study is innovative because a group of healthy volunteers were included, and serum ROS and caspase-3 levels were obtained from them.
It was noted that there were no differences in systemic ROS levels between healthy volunteers and septic patients, and no differences between septic patients who died ICU and those who survived. Moreover, there were no differences between patients who died during the first day of hospitalization and those who survived. These results are not in accordance with those obtained in previous studies performed on rats, in which the septic group had higher ROS levels than the control group, using the same technique to measure ROS [35]. These differences may be due to methodological issues because, at the time of sample collection, the rats had had sepsis for 24 h, while patients in the hospital came in through the emergency service, they were supported with fluids, the first dose of antibiotics was administered, then they were admitted to the ICU, a central line was placed, they were intubated if necessary, vasoactive drugs were administered, and finally the samples were taken. Another possible cause for this difference between the results obtained from rats and those from patients is that they are different models, with a different pathophysiologies, and therefore it is often difficult to replicate animal results in patients [15, 29].
This study is innovative because serum ROS were measured in septic patients instead of oxidative stress markers. Previous literature associated damage at the tissue level with ROS [36–41]. Using the current technique, ROS levels in yeast were measured under stress conditions with Menadione [42]. The DCFH technique is an already described technique to measure intracellular ROS also in extracellular fluids [43–46]. It has been demonstrated in our laboratory that septic rats have higher levels of ROS than controls, and that the administration of parenteral succinate decreases ROS levels [35].
In several models, it has been shown that ROS damage cell structures including DNA, which leads to apoptosis. The presence of serum ROS in an animal model of sepsis are also increased, but this is not associated with markers of organ failure [47]. Lorente et al. [48] showed that the total antioxidant capacity of septic patients who did not survive was higher and, in turn, this was a marker of 30-day mortality. Also, septic patients with a SOFA score higher than 7 had higher ROS levels measured with DCFH [49].
DCFH emission results and corresponding serum ROS levels in septic patients are not in accordance with studies that measure oxidative stress, probably because different techniques are used. In this study, DCFH emission that states serum ROS levels was measured, while in other studies, either antioxidant capacity or oxidative stress substitutes, such as TBARS or oxidized proteins, were measured.
On the other hand, apart from the measurement of ROS and oxidative stress markers, multiple studies with antioxidants have been carried out. Preclinical and clinical trials with antioxidants had diverse results. In a preclinical murine model of sepsis, circulating cytokines, increased urea, ALT, and LDH, decreased glucose, lymphopaenia/neutrophilia, and NO blood release were not altered with the treatment with SkQ1 antioxidants, and there was no difference in survival with antioxidant mtAOX [50]. Other preclinical trials had positive results. Superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) activities were decreased after the administration of antioxidant quercetin in septic rats [51]. In rats that were injected intraperitoneally with lipopolysaccharide, it was observed that pretreatment with N-acetylcysteine (NAC) decreased certain parameters of oxidative stress [52, 53]. Also, in a study with septic rats, the administration of intraperitoneal NAC, associated with deferoxamine, decreased the production of superoxide, the activity of enzyme catalase, superoxide dismutase, and myeloperoxidase in all organs, in addition to improving survival [54]. In several clinical trials in which septic patients were supplemented with antioxidants, the length of mechanical ventilation or oxygen levels were not reduced [55–68]. There are many works that describe differences between animal models of sepsis and patients, and the difficulty of transferring the results of the animal model to patients [69–71]. The absence of positive results in clinical studies with antioxidants has some sort of relationship with the results of the current study, in which there were no differences between the ROS levels of healthy volunteers and septic patients.
In addition, septic patients had higher serum levels of caspase-3 than healthy volunteers, with statistically significant differences. The median serum levels of caspase-3 in patients who died was 75% higher than in those who survived, but the differences were not statistically significant. Similarly, the median of patients who died on the first day was 90% higher than those who survived the first day, but these differences were not statistically significant. This is due to the statistical test chosen and the spread of data with a large confidence interval width [72], but it does not make the data less relevant [73].
When comparing the data obtained with those from the literature, there are certain similarities. In a study carried out by Lorente et al. [74] a higher mortality in septic patients with higher serum levels of caspase-3 measured using the ELISA technique was shown. In this study, higher caspase-3 levels are associated with higher mortality (odds ratio, 6.51; 95% CI: 3.32–12.77; P < 0.001) [74]. In the same study, a positive correlation between caspase-3 and a different marker of apoptosis, caspase-cleaved cytokeratin-18, was found [74]. The same group published a study in which caspase-cleaved cytokeratin-18 was higher in septic patients who died in the ICU and had a positive correlation with levels of interleukin 6, APACHE II, SOFA, and lactate [75]. Similarly, this research group found that higher levels of caspase-cleaved cytokeratin-18 (levels over 20 UI L-1) resulted in higher mortality (OR = 8.476; 95% CI: 2.087–34.434; P = 0.003) [76]. Although these results contrast with those obtained in the present work, it should be taken into account that patients were different, and the laboratory tests to measure serum caspase were different. These are possible explanations for these differences in the results.
Also, this study is innovative because it was noted that there was no correlation between serum levels of ROS and caspase-3 between healthy volunteers and septic patients. Nor was there a correlation between serum levels of ROS and caspase-3 when analysing only the patient group. This is an interesting finding, which suggests that there is no association between those molecular processes in sepsis. No other study in the bibliography that quantified ROS and apoptosis markers was found. ROS are molecules with a very short life. A limitation of this study is that samples were taken in the ICU, after a central line was placed and vasoactive drugs were indicated. More studies with samples taken at hospital admission and different times of stay should be performed to clarify this finding.
Finally, it should be clarified first that, although the recruitment time was relatively long, the conditions remained the same during the study and all patients were treated by the same medical team, although the therapies could have changed slightly in that period. Second, only ROS were measured in serum; other measurements could have been carried out (i.e. carbonylated proteins or TBARS), but the objective was to observe the effect of the relationship between these chemical species and mortality, as well as their relationship with apoptosis markers. Finally, it should be considered that the severity of the patients’ disease was very heterogeneous, because obtaining the largest possible number of cases was deemed more important than the homogeneity of the sample. A more homogeneous sample could have generated other results.
CONCLUSIONS
In the present work, serum ROS levels were measured, and septic patients did not show higher serum ROS levels than healthy volunteers. Conversely, patients showed higher serum levels of caspase-3 than healthy volunteers. There was no correlation between the serum levels of both markers. These results would indicate that serum ROS do not serve as markers of oxidative stress in septic patients, despite the fact that ROS production is known to take place at the tissue level, but instead, serum apoptosis markers could reveal the occurrence of cellular damage and consequent multi-organ failure in these patients relatively fast and with ease.




