Coronavirus disease 2019 (COVID-19) is associated with respiratory impairment by multifocal pneumonitis. The most severe expression, when acute respiratory distress syndrome (ARDS) is present, is termed CARDS (COVID-19-ARDS). Prolonged prone positioning (PP) is an established treatment for patients with ARDS [1–4]. PP provides various effects leading to pulmonary recruitment and improvement of oxygenation [3].
Most patients require analgesia and sedation to tolerate prolonged PP. Even in the supine position control of dyspnoea when applying “lung protective” ventilation parameters is facilitated and may result in desperate “air hunger” in COVID-19 patients [5]. Analgosedation seems to be difficult in COVID-19 patients with high requirements of sedatives [6]. Whilst experts traditionally prefer intravenous substances, inhalational volatile sedation is increasingly promoted also in CARDS as accumulation is less likely with inhaled sedatives and sedation depth is easier to control [7, 8].
In our tertiary care ARDS centre we traditionally favour volatile sedation maintained with inhaled sevoflurane for patients during prolonged PP. Adjustment of desired sedation depth is effective and this regimen even allows assisted spontaneous breathing (ASB) during PP for patients with ARDS. Our protocol has been published previously [9]. During treatment of our first patients with CARDS we observed that our former protocol had shortcomings regarding sedation depth. Patients needed excessive doses of sevoflurane to tolerate PP and self-endangering agitation was observed. We then combined intravenous esketamine to inhaled sevoflurane and immediately found sufficient sedative depth and sustained respiratory drive. This study was designed to evaluate the feasibility and safety of our sedation regimen in COVID-19 ARDS. We hypothesized that inhalative sedation in combination with esketamine is a safe sedation regimen for COVID-19 even allowing ASB during PP.
METHODS
Patients were recruited in a German University ARDS centre endowed according to international recommendations and certified by the German Society of Anaesthesiology and Intensive Care Medicine (DGAI) [10]. Patients with severe CARDS were treated according to a departmental standardized protocol. Patient data were anonymously collected. The study was approved by the local Ethics Committee (EK 235/20) and the general contract governing medical treatment. It was confirmed that no further informed consent was necessary because of the descriptive, non-interventional and anonymous design of the study and the special circumstances during the COVID-19 pandemic [11]. The study was planned and designed in accordance with the initiative for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), using the suggested checklist for epidemiological cohort studies [11].
Patients
All mechanically ventilated COVID-19 patients admitted to the departmental intensive care unit (ICU) between 12th March and 9th June 2020 were included in the study. Patients with non-invasive ventilation or patients not suitable for PP were excluded from the analysis.
Analgosedation
The protocol was inspired by Heider et al. [9]. Sedation was established by inhaled sevoflurane (Sevoflurane Baxter, Baxter Germany GmbH, Unterschleissheim, Germany) continuously applied with the AnaConDa-S-System (Sedana Medical AB, Danderyd, Sweden). Target end-tidal concentration was between 0.5 and 1.3 Vol% depending on sex and age of the patient aiming for an xMAC (minimal alveolar concentration) of 0.4–0.6. The sedation target was a value of –4 on the Richmond Agitation and Sedation Scale (RASS) [12]. Patients also received 10–20 mg of oxycodone in a slow release preparation twice a day via a nasogastric tube.
Intravenous esketamine was additionally applied continuously with 0.2–0.4 mg kg–1 h–1. As the visual analogue scale (VAS) cannot be applied in sedated patients in PP, criteria for additional piritramide – a low potent intravenous opioid – application were vegetative symptoms of stress like tachypnoea, tachycardia, sweating, body motion, etc.
Prone positioning and ventilator settings
PP was applied in all patients with CARDS. PEEP was fixed between 12 and 16 mbar. Inspiratory fraction of oxygen (FiO2) was set aiming for an arterial oxygen partial pressure (PaO2) of ≥ 60 mm Hg. Patients were converted in the ASB mode “CPAP-ASB” of the Evita Infinity V500 respirator (Drägerwerk AG & Co. KG, Lübeck, Germany). Criteria for reconversion to pressure-controlled ventilation (PCV) were respiratory acidosis with blood pH < 7.2. Esketamine and opioid application was reduced and when patients started to breathe spontaneously, the respirator was again switched to CPAP-ASB mode. If spontaneous breathing could not be established promptly, naloxone was administered in 0.04 mg doses at the discretion of the responsible nurse. Nurses were advised to aim for tidal volumes of ≤ 6 mL kg–1 predicted body mass (males = 50 + 0.91 [height (cm) – 152.4] kg; females = 45.5 + 0.91 [height (cm) – 152.4] kg) by modification of supportive pressure, opioid application and end tidal sevoflurane concentration.
Definitions
Unexpected events were divided into severe, moderate and mild events. Severe events were self-extubation or self-endangering movement with need for conversion from prone to supine position. A moderate event was defined as an episode in which the responsible nurse quoted “insufficient sedation” with need for intervention. Mild events were for example hypercapnia resulting in conversion from CPAP-ASB to PCV.
Horovitz index
The Horovitz index (HI), which is the PaO2/FiO2 ratio (mm Hg), is an indicator of the extent of an oxygenation disorder [13].
Types of CARDS
Two phenotypic patterns of CARDS have been described: the L-type with low lung elastance, low lung weight and low response to PEEP; and the later H-type which basically seems to be more similar to “conventional” ARDS [5, 6]. Patients with H-type CARDS therefore may also benefit from prolonged PP [7, 8].
Data collection and statistics
Detailed data about the ventilation mode, hemo-dynamic parameters, RASS and sevoflurane consumption were drawn from the electronic chart (Copra 6, Copra Systems GmbH, Berlin, Germany). Reports on unexpected events and the patient’s condition were documented every 8 hours by both nurses and intensivists in the electronic chart. Data were collected in Microsoft Excel (Microsoft, Redmond, Washington, USA). The GraphPad InStat software (GraphPad Software Inc, La Jolla, California, USA) was used for statistical analyses. Differences between patients with compliance < 50 mbar mL–1 and compliance > 50 mbar mL–1 were explored using the unpaired t-test and Welch test.
End points
The primary endpoint was the incidence of sedation-related unexpected events. Secondary endpoints were length of spontaneous breathing related to duration of PP and comprehensive respiratory parameter.
Ethics approval and consent to participate
Demographic and specific laboratory details of the patients were prospectively anonymously collected in a local CARDS registry. Protocol based CARDS therapy is covered by the general contract governing medical treatment. The local ethics committee approved the analysis and publication of the data and confirmed that no specific patient’s consent was necessary because of the anonymous and observational design of the study and the special circumstances during the pandemic (EK 235/20).
RESULTS
Patients
15 out of 19 consecutive COVID-19 patients between 12th March and 9th June 2020 were intubated and included in the study. Detailed patient characteristics are listed in Table 1.
TABLE 1
Patient characteristics (N = 19)
Primary endpoint – complications and unexpected events
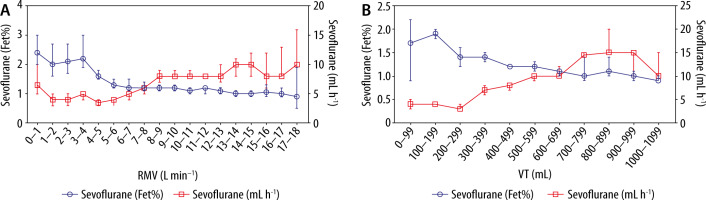
No severe event occurred during sedation with inhaled sevoflurane and intravenous esketamine. Only one patient had leakage of the endotracheal tube in PP, but the tube could easily be advanced for a few cm in PP, which solved the problem. 15 events of “insufficient sedation” were documented in 9 different patients. 14 of them (93%) were due to low tidal volumes resulting in low sevoflurane levels. All of them could be solved by conversion from assisted ASB to PCV respirator mode. 5 events (26%) interestingly occurred during high respiratory minute volumes (RMV). Despite high sevoflurane delivery rates from the syringe pump, end-tidal sevoflurane levels were not adequate. This can be traced back to technical limitations of the AnaConDa-S System [14] (Figure 1).
FIGURE 1
Inhaled sedation with the AnaConDa-S System (Sedana Medical AB, Danderyd, Sweden) becomes less effective when minute volume exceeds 7–8 L min–1, most likely due to ineffective sevoflurane recondensation
4 mild events were observed: patients had to be converted from ASB to PCV because of hypercapnia or hypoxia.
Duration of assisted spontaneous breathing
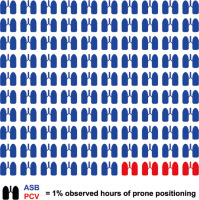
Overall, 2610 hours of PP have been analysed. During 2498 hours (96%), patients were successfully converted to a pressure-supported spontaneous breathing mode. The median continuation of each prone episode was 17 hours (IQR 1.00). Median continuation of pressure-supported spontaneous breathing per episode was 16.5 hours (IQR 1.00) (Figure 2).
Improvement of oxygenation during prone positioning
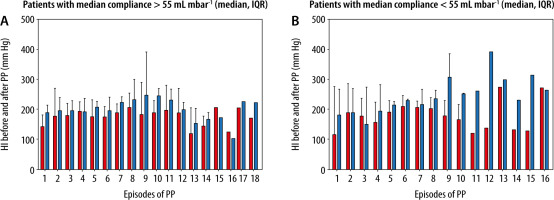
When compliance was < 55 mbar mL–1 patients (“H-Type COVID-19”) showed a significant increase of the HI after conversion to PP. In addition, patients with a median compliance < 55 mbar mL–1 improved over time from episode to episode of prone PP (Figure 3).
Reason for evolving our sedation regime
Many patients examined showed an increased need for sedatives and a high respiratory minute volume (RMV). Mono sedation in these patients with inhalative sevoflurane obviously is difficult. This may be due to technical limitations of the AnaConDa-S device at high RMV [15, 16] (Figure 1).
In combination with esketamine, conversion to pressure-supported spontaneous breathing during PP was feasible in 2498 hours from 2610 hours in PP (Figure 2). No adverse psychomimetic side effects were observed with the additional administration of esketamine to inhaled sedation with sevoflurane.
Reasons for failed conversion to assisted spontaneous breathing
The treatment of the patients was changed to pressure-controlled ventilation at an arterial blood pH < 7.2. Seven patients were intubated and turned into PP upon arrival. Eight patients experienced accumulation of opioids and sedatives they had received prior to administration to the ICU. Therefore, it was not possible to immediately convert these patients to pressure-supported spontaneous breathing. In conclusion, we identified two main factors for failed conversion to spontaneous breathing in PP: muscle relaxation and sedation with opioids.
DISCUSSION
Main findings
This observational study demonstrates the feasibility and safety of analgosedation with inhaled sevoflurane in combination with intravenous esketa-mine during PP in intubated CARDS patients. No severe sedation-related event was observed during 146 episodes of PP with 2610 ventilation hours. Moreover, an assisted spontaneous breathing mode could be established during 96% prone duration (Figure 2).
Comparability to other studies
To our knowledge, these are the first data evaluating the safety of the sedation regimen we used and assisted spontaneous breathing in PP during CARDS. The decision between controlled mandatory ventilation and assisted spontaneous breathing modes in respirator therapy of CARDS is still controversial [9, 19]. ASB decreases intrathoracic and driving pressure, recruits atelectatic areas of the lung, supports right ventricular function and prevents patients from ICU-acquired muscle weakness in ARDS [12, 20, 21]. The risk of increased transpulmonary pressure, desynchronization and consecutive risk of ventilator-induced lung injury (VILI) [22, 23], however, may support controlled ventilation, especially in L phenotype of CARDS, to achieve “lung protection” [24]. Lung protective controlled ventilation in combination with insufficient sedation on the other hand may amplify ventilator desynchronization and cause the desperate feeling of “air hunger”. In almost all studies focusing on treatment of ARDS with PP, deep sedation, usually for 24 hours, was used in combination with controlled ventilation [25]. In contrast, there is clear evidence that deep sedation has a relevant effect on acquired muscle weakness in intensive care, survival, and quality of life after hospitalization [26, 27].
Crotti et al. [28] in their cohort with “non-COVID” ARDS found only a few patients with ARDS able to breathe spontaneously when sedated intravenously. In contrast to this finding, in the present study, ASB was achieved within more than 90% of the cumulative time in PP. Even in combination with intravenous esketamine, inhaled sevoflurane had hardly any respiratory depressant effect and thus enabled spontaneous breathing and sufficient sedation depth during PP.
Clinical significance
For our cohort we decided in favour of volatile sedation, as we wanted to avoid the negative side effects of benzodiazepines, alpha-2 agonists or propofol. Benzodiazepines, even when applied in a “low dose”, cause delirium and cognitive dysfunction and have been shown with good evidence to be independent risk factors for the development of post-traumatic stress disorder (PTBS) and acquired muscle weakness following ICU treatment [29, 30].
Alpha-2 agonists have an unfavourable side-effect profile [32, 33] and, like clonidine, have long half-lives or, as in the case of dexmedetomidine, often do not achieve adequate depth of sedation at the recommended maximum dose [31].
Although infusion of propofol is common in the ICU and dosing seems simple, it has significant disadvantages such as risk of propofol infusion syndrome, and especially in patients with COVID-19 propofol turned out to be associated with complications [34].
Inhaled sevoflurane in contrast provides sufficient sedation during PP and fast awakening that allows the patient to actively participate in his therapy and recovery. Besides a well-controllable sedative effect, sevoflurane provides a number of side effects that seem to be beneficial for the treatment of patients with CARDS [35–37]: These include bronchodilating [38], anti-inflammatory [39] and, in theory, cardioprotective effects [40, 41]. However, cardioprotective effects have not yet been clinically demonstrated [42].
We observed problems with inhaled sedation, particularly in early L-type COVID-19 disease patients with high compliance, high tidal volumes, and high RMV. We used the AnaConDa-S-System (Sedana Medical AB, Danderyd, Sweden) to perform inhaled sedation. At high respiratory minute volumes of more than 7–8 L min-1, this system reaches its technical limits [14–16], and it is more difficult to achieve high Fet% rates. The reflector loses efficiency at high tidal volumes. The capacity of the reflector is exceeded when more than 10 mL of anaesthetic vapour is contained in an exhalation train (Figure 1).
Compared with our conventional ARDS patients, patients with CARDS showed an increased need for sedative medications. Less pronounced septic encephalopathy may be one of the reasons for the high need for sedatives in patients with CARDS [43], although there is increasing evidence for cerebral involvement in COVID-19 [44].
Many of our patients presented with high fever leading to an increase in the MAC of sevoflurane and thus an increased need [17].
Since the combination with opiates often led to apnoea and controlled ventilation, we used intravenous esketamine to achieve the desired depth of sedation.
Coanalgesic effects contributing to opioid reduction, maintenance of spontaneous breathing, bronchodilatation, and ubiquitous availability are desirable characteristics for this sedative. In addition, esketamine leads to more stable haemodynamics with reduced need for vasopressor therapy due to endogenous catecholamine release. On the other hand, psychotropic effects, hypersalivation, and possible liver damage are unfavourable properties for long-term use of esketamine [45]. To prevent undesirable psychomimetic effects from esketamine administration, esketamine should not be used exclusively. The application interval of esketamine should therefore be optimally matched to the application interval of sevoflurane.
Limitations
As the ICU and hospital were well-prepared for COVID-19, there was no shortening in provider resources. Therefore, all patients received therapy according to the departmental standard operating procedure (SOP), which could be adapted to the current situation by an experienced team. Data and side effects of therapy were documented thoroughly. A limitation of our study is the monocentric design and the lack of a control cohort. It would be favourable to evaluate these findings in a larger cohort of patients. The AnaConDa-S device increases the dead space by approximately 50 mL, which can promote the development of respiratory acidosis.
CONCLUSIONS
Inhaled sedation with sevoflurane in combination with esketamine safely enables PP in CARDS. Inhaled sedation with the AnaConDa-S -System becomes less effective when minute volume exceeds 7–8 L min–1, most likely due to ineffective sevoflurane recondensation. By adding esketamine to inhalative sevoflurane even spontaneous breathing in PP can be maintained and the many beneficial effects of inhaled sedation can be further exploited. Whether this concept is advantageous compared to established ventilation and sedation strategies must be evaluated in a larger cohort in the future.