Introduction
Atopic dermatitis (AD) is a condition which highly affects the children presenting with chronic and recurrent skin lesions with pruritus. These lesions highlight clinically several pillars of its pathogenesis such as the loss of skin barrier integrity, microbiome dysbiosis, changes in the innate and adaptive immune responses [1]. The damage in the skin barrier is caused by changes in stratum corneum lipid composition, loss-of-function mutation in the filaggrin gene and additionally, disruption of host microbiota.
The contribution of microbiota to human health and disease still remains entirely unexplored. It is known that microorganisms creating the human microbiome are necessary for the proper functioning, including maintaining the immune status of the macroorganism [2, 3]. Currently, the term “microbiota” means all of the microorganisms, i.e. bacteria, fungi, viruses and archaea, inhabiting ecological niche [4]. Overall, microbiota can form functionally complex structures, capable of communicating with each other and with the host.
The microbiome is altered in AD. During flares there is an increased count of Staphylococcus aureus (S. aureus) and reduced microbiome diversity. The most known pathogen aggravating inflammatory lesions is S. aureus. Its colonization on both lesional and non-lesional skin prove importance in severity of AD [5]. S. aureus expresses molecules including δ-toxin, α-toxin, protein A, superantigens and lipoproteins that may cause direct cell damage triggering inflammatory response [6].
The inhibition in production of antimicrobial peptides in the defected AD skin can lead not only to bacterial but also viral and fungal infections [7]. Lately, a possible role of Malassezia species has been suggested in exacerbation of skin lesions. These fungi activate Th2 immune response in the disrupted epidermal barrier via human dendritic cells or keratinocytes, which exacerbate skin lesions in AD [8]. It is recommended to use antifungal agents in the coexisting fungal infection on AD skin. On the other hand, bacterial infection is managed with both antibiotics and antiseptics. Due to the growing problem with bacterial resistance to antibiotics, antiseptics seem to be a safer alternative in therapy. Topical use of dyes could be an interesting option for antimicrobial measures. One of the substances that has been used since the 19thcentury is gentian violet (GV). It is known for its antibacterial, antifungal, antihelminthic, antitrypanosomal and antiviral activities. Claims of GV efficacy have been widely visible in treatment of many diseases especially in dermatology for example in impetigo [9], wound healing [10], paronychia, thrush, angular cheilitis or umbilical infection [11]. However, some of the data show evidence of genotoxic and carcinogenic mode of action in the mouse and rat studies [12]. Lately, the Food and Drug Administration (FDA) has prohibited to use GV in animal feed [13]. The FDA has issued alerts for domestic and imported seafood for GV content from a number of countries. Nevertheless, human testing on the mutagenic effect of gentian violet is sparse. The clinical practice has shown long-time observation of its effectiveness in topical use of 1–2% solution of this dye. This study aims to evaluate the influence of 2% GV aqueous solution on the selected bacteria and fungi on AD skin.
Aim
The aim of this study was to assess changes of skin microbiome after topical 2% aqueous GV use in patients with atopic dermatitis and the control group. We also analysed microbial composition in AD subjects before the treatment.
Material and methods
All parents or guardians of the subjects gave written informed consent prior to participation. For the study, 60 participants (2–12 years old) were voluntarily recruited. Recruitment of the patients with atopic dermatitis took place in the Department of Dermatology, Paediatric and Oncologic Dermatology, Lodz Medical University. The first 30 patients were hospitalized at the ward due to AD. The other 30 controls (healthy subjects) were selected randomly during the same period as AD patients and matched for age, gender and ethnicity. Exclusion criteria were the same as those applied to patients with AD. Subjects were excluded from study participation if they used topical or systemic glucocorticosteroids for 2 weeks, topical or oral antibiotics for 2 weeks or any immunosuppressive and immunomodulatory treatment prior to the study. Patients with neoplasms or active infectious disease were excluded as well.
Thirty patients with AD were diagnosed according to the criteria of Hanifin and Rajka. Clinical evaluation of severity of the atopic eczema was measured with the Scoring Atopic Dermatitis (SCORAD), Eczema Area and Severity Index (EASI) and Investigator Global Assessment (IGA) index. The patients were screened regarding the onset age, duration of disease, and the number of exacerbations during the year.
The material for microbiological testing was collected from eczematous skin lesions on antecubital fossae by using 25 cm2 impression plates. The agar surface was pressed against the designated lesions with the same pressure. The impression plates have convex surfaces that adhere closely to the tested skin area. Furthermore, 2% aqueous GV was applied on the decubital fossa. The dye was used twice daily for the same area. No emollients were applied on the examined skin area during the study. After 3 days, the impression plate adherence was repeated for the same region. Two types of plates were used: CHROMagar Staph aureus and CHROMagar Malassezia. The first chromogenic medium determined the count and initial identification of aerobic Gram-positive cocci. CHROMagar Malassezia enabled the occurrence and isolation of fungi. Both types of media were incubated according to the manufacturer’s instructions and microbiological standards: 48 h at 35.5°C. The media detecting fungi were analysed, followed by prolonged incubation for another 48 h in case of absent strains. On the basis of the pigmented phenotype, both bacterial and fungal colonies grown were counted. Numbers were expressed as CFU (colony forming unit) per 25 cm2.
The Phoenix BD apparatus was used for the identification of cultured microorganisms.
Statistical analysis
For statistical purposes, 60 patients in the study were divided into four subgroups as follows:
– Subgroup 1: AD patients before the application of 2% aqueous GV,
– Subgroup 2: AD patients after the application of 2% aqueous GV,
– Subgroup 3: control group before the application of 2% aqueous GV,
– Subgroup 4: control group after the application of 2% aqueous GV.
The Shapiro-Wilk test was used for the evaluation of normality. The differences between the groups were assessed using Mann-Whitney U test. Statistically significant differences between the subgroups were presented graphically in the charts. The Kruskal-Wallis test was carried out to check the differences between the four study subgroups. If the result of the Kruskal-Wallis test was statistically valid, the Dunn test was additionally performed. Spearman’s correlation was used to find relationships between the number of bacteria and other continuous variables. Significance levels for all analyses conducted were p < 0.05. The Statistica software (v.6.0 Statsoft, Tulsa, OK, USA) was used for statistical analyses.
Results
Sixty patients (30 with AD, 30 control group) aged from 2 to 12 years, were enrolled in the study (51.67% were boys). The median age of AD patients was 6.8 years and in the control group 8.7 years (p > 0.05). The median AD duration was 2.95 months. All patients at the time of enrolment had moderate skin symptoms; the median IGA score was 3 (2–4), EASI was 11.4 (3.4–26.5) and SCORAD was 45.9 (19.3–68.6). Low-potency corticosteroid medicinal products prepared on the basis of a magistral formula were commonly used in 83.33% of cases as initial therapy on all eczematous areas at least 2 weeks prior to the study enrolment. The most common medicinal product applied by the patients was 1% hydrocortisone ointment.
In all 60 patients we have identified bacterial species such as Staphylococcus spp., Micrococcus spp. and bacteria of the Gram-positive family (Tables 1 and 2). In both groups Malassezia spp. and Staphylococcus spp. were present on the skin. However, in the AD group there was a noticeable diversity in strains of Staphylococcus spp.
Table 1
Skin microbiota changes before and after application of the gentian violet in AD patients
Table 2
Skin microbiota changes before and after application of the gentian violet in the control group
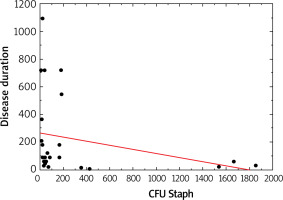
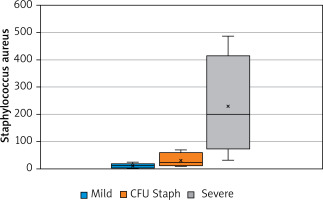
Moreover, Staphylococcus spp. was associated with the duration of disease (p > 0.05, Figure 1). The number of S. aureus on lesional skin positively correlated with the severity of disease according to validated scoring systems such as IGA, EASI, SCORAD (p < 0.05, Figure 2). However, there was no relation between S. aureus count, severity of disease and age (p > 0.05).
Figure 2
Relationship between the severity of the disease (according to scoring systems – IGA, EASI, and SCORAD) and the number of Staphylococcus aureus CFU (p < 0.05)
Total bacteria counts were statistically decreased after GV treatment in both groups (p < 0.05). The significant reduction in the number was seen in Micrococcus spp. (M. luteus, M. lylae), Staphylococcus spp. (S. aureus, S. capitis, S. haemolyticus, S. cohnii) in AD patients. The Kruskal-Wallis global test was used in all 4 study subgroups to determine the differences in the number of each bacterium. It was observed that the Staphylococcus spp. population was decreased in the all subgroups (p < 0.0089). Dunn’s test pinpointed a statistically significant difference in the number between subgroup 1 and 3 (p < 0.0132), while the Staphylococcus spp. count was maintained in subgroup 2 and 3 (p = 1.000) (Table 3).
Table 3
Correlation between CFU of Staphylococcus spp. in 4 study groups (p-values)
| Subgroup 11 | Subgroup 22 | Subgroup 33 | Subgroup 44 | |
|---|---|---|---|---|
| Subgroup 11 | 0.3225 | 0.0132 | 0.0042 | |
| Subgroup 22 | 0.3225 | 1.0000 | 0.8685 | |
| Subgroup 33 | 0.0132 | 1.0000 | 0.8859 | |
| Subgroup 44 | 0.0042 | 0.8685 | 0.8859 |
All the subjects included in the study tolerated the treatment very well. There were no side effects observed.
Discussion
GV as a triphenylmethane rosaniline dye has been known for decades. The pioneer study evaluating its bactericidal and fungicidal property was reported in 1912 by Churchman [14]. In animals, GV has been used for treatment of fungal and parasitic infections in fish and topically for eye infection in livestock [15]. The antimycotic properties were also proven in several studies. It has been demonstrated that GV lowers the adherence of Candida spp. to the catheters [16]. A randomized unblinded study on the treatment of oropharyngeal and oesophageal candidiasis that was conducted among 141 patients with AIDS and oropharyngeal candidiasis showed comparable effectiveness of GV to ketoconazole [17]. Even though numerous studies were conducted, there were no data of its influence on Malassezia spp. This genus of yeasts is attributed with both commensal and pathogenic role in AD. Jensen-Jarolim et al. isolated these yeasts from eczematous lesions on the neck and head [18]. The reason why these yeast were present on eczematous lesions might be their overexpression of phospholipases and lipases [19]. They could damage the epidermal barrier in AD patients inducing a pro-inflammatory immune response by the skin and immune cells. In spite of natural attraction towards seborrheic areas and skin folds, our study showed limited strains of Malassezia spp. in decubital fossa but it was below a statistical significance threshold. The condition could find the reason in excessive dryness of the skin lesions and bacterial flora domination. Our experiment shows that after GV exposure there was no growth of the Malassezia population on AD skin, with a reduction in the number of colonies in the control group. A small number of patients included in our study and single colonies isolated from eczematous lesions do not allow to draw unequivocal conclusions about the antifungal activity of GV on Malassezia spp.
The antibacterial action of GV has been more acquainted [20]. The most common hypothesis suggests the inhibition of bacterial cell wall formation, glutamine synthesis suppression by blocking glutamic acid metabolism in the pathogens, protein synthesis blockade due to the dye binding to ribosomes, alteration of the redox potential or targeting NADPH oxidase and thioredoxin reductase 2 in microbial cells [21]. In the literature there are several studies describing positive bactericidal properties of the dye. Brockow et al. showed the reduction of S. aureus density after applying 0.3% aqueous gentian violet in AD patients [22]. The results of this study are in accordance with the authors’ observation that faster clearance of AD is achieved, when GV is added to a potent topical corticosteroid. This combination might be helpful in minimizing side effects of steroids. In our study, we discovered a significant S. aureus reduction after 3 days of 2% aqueous GV application.
It has been demonstrated that the microbial composition of AD skin dominates in S. aureus in comparison to healthy skin [23]. Our investigation confirmed that before GV application, there was a significant number of S. aureus on AD skin. S. aureus was positively associated with disease severity. Moreover, there was an inverse correlation between the S. aureus number and the length of disease. This may contribute to enhancement of flare development and skin barrier impairment progression caused by S. aureus in the early AD phase. In contrast, Totte et al. noticed no relation between S. aureus and AD severity in children aged 0–18 years [24]. The sites of flares in AD children vary, depending on age, which could inflict the differences in the results of the study. Our AD population comprised only 30 subjects aged 2–12 years, yet the samples were collected from decubital fossa, the most common affected site for this age group. Next to severity, age of the AD patients did not show any correlation with the number of S. aureus. There was a number of studies investigating the skin microbiome in AD in different age groups. Meylan et al. discovered S. aureus density remarkably greater on axillary and decubital fossa at the time of diagnosis in AD children under the age of 2. However, it was significantly lower than in older AD population [25]. On the contrary, Zheng et al. found similar bacterial microbiome on the same area in infants with AD and age-matched healthy controls [26]. A more consistent study in anatomical site sampling is needed on a larger population to estimate the age-specific risk factor for S. aureus in AD.
It has been described that the S. aureus virulence is enhanced by the native and commensal microbiome of the skin and individual species acting as “pro-infective agents” [27]. The bacteria which were considered to be the permanent microbiome of the skin are Micrococcus spp. [28, 29]. In 2018 Boldock et al. showed that Micrococcus luteus (M. luteus) [27] enhanced the activity of S. aureus in a mouse model [30]. The study proved that commensal bacteria may actually help pathogens evade the immune system. This discovery prompted scientists to investigate components of the M. luteus cell wall, such as peptidoglycan, for their ability to influence hosts’ immunity. In an animal model, the authors showed that the polymer also exacerbated infection with both drug-resistant and drug-sensitive strains of S. aureus. The researchers also found that M. luteus secreted a small resuscitation-promoting factor (RPF) protein, which stimulated the growth of bacteria such as Staphylococcus spp. An “extended infection” hypothesis was formulated to define the role of resident environmental microflora in disease caused by an invasive pathogen. In the light of the above, we believe that under the specific conditions in damaged AD skin, multispecies biofilm may appear. We suggest that under these circumstances M. luteus can protect S. aureus by “hiding” it from the effects of targeted drugs or bacterial factors. In our study GV decreased the number of Gram-positive bacteria and resulted in the bacteria reduction to the level in which the biofilm was either destroyed or significantly damaged. To confirm this hypothesis, it is necessary to conduct a study on a larger group of patients who would be repeatedly tested both microbiologically and immunologically [30].
The population of Staphylococcus spp. includes at least 40 species that inhabit both healthy skin and AD skin. Our study revealed a significant diversity in staphylococcal composition in comparison to healthy subjects. We noticed S. aureus, S. warneri, S. capitis, S. haemolyticus, S. cohnii, S. simulans, S. equorum, S. pasteuri, S. epidermidis and S. lugdunensis on the antecubital area of the AD skin. Many studies show the possible bactericidal role of Staphylococcus epidermidis, S. lugdunensis and S. hominis in limiting the number of the S. aureus in AD [31, 32]. Moreover, a recent paper of Cau et al. suggested that S. epidermidis can damage the skin similarly to S. aureus by production of cysteine protease [32]. Another study group observed co-colonization of S. capitis and S. lugdunensis in the decubital crease of adult AD skin, correlation with the disease severity and proportional reduction in the number of S. hominis and S. cohnii [33]. The number of bacteria in this study was estimated in adult population, which can differ from paediatric patients.
Our results indicated a decrease in the count of not only S. aureus but also S. capitis, S. haemolyticus, S. cohnii and S. kloosii in AD patients after GV exposure. Additionally, we have discovered a comparable number of Staphylococcus spp. between the AD group after the GV treatment and the control group before the GV treatment. This may suggest that GV does not damage the skin surface ecosystem and allows the reduction of excessive bacterial counts to a “safe” level, i.e. close to that of healthy children. Disruption of the resident microbiota of the skin is often seen after the use of antibiotics. SanMiguel et al. have stated that topical antibiotics elicit shifts to resident skin bacterial communities and reduce colonization by S. aureus competitors [34]. They found that both antiseptics and antibiotics decrease colonization by commensal Staphylococcus spp. However, antiseptics elicited only minor changes to skin bacterial populations, with few changes to the underlying microbiota. A similar effect was represented by GV in our study.
GV is normally used in concentrations ranging from 0.5% to 2%. The toxicity of the dyes in higher concentrations (1% or 2%) was observed in the literature [35]. No side effect after GV use was observed in either of our studied groups. The GV is available in two forms: aqueous and spirit solution. In our research we used a concentration of 2% in aqueous solutions. However, it should be applied with care on skin folds, preferably in a lower concentration due to the risk of necrotic reactions [36]. GV-induced toxicity including carcinogenicity and genotoxicity has been documented in the scientific literature [15]. But only one of the published studies was conducted using the topical route of exposure. In a prospective study analysing the effects of methylene blue and violet gentian in dressings for the treatment of chronic wounds and local infections it was found that the use of Hydrofera Blue Dressing did not increase the risk of developing cancer [37]. The intensity of the carcinogenic effect in animals, induced by GV, has been noted as dose-dependent [38]. When the dye is applied topically on large surface areas and muco-cutaneous lesions, systemic absorption may occur. In the light of the above, we suggest avoiding oral exposure to the substance and not using it on a large body surface. Our research aimed to screen the mechanism of GV action towards skin microbiome representatives such as Staphylococcus spp., Micrococcus spp., and Malassezia spp., not for its mutagenic activities. As for many new therapeutic options in AD, GV may be used as an alternative antibiotic-sparing therapy but with caution.