Introduction
Neoplasms are the second cause of death after diseases of the cardio-vascular system among transplant recipients (OTRs). With the help of modern immunosuppressive treatment transplanted organs function well for many years. However, in the course of time, complications from immunosuppressive treatment may be observed. One of the most fundamental complications of this treatment is an increased risk of cancer development [1–3].
Skin cancer is the most frequently described neoplasm among OTRs, whereas squamous cell carcinoma (SCC) is very often declared to be the most frequently occurring cancer among those patients. According to some authors, the frequency of the SCC occurrence is estimated to be between 1% and 6.5% within first 5 years after the transplantation and from 6% up to 35% within 10 years post transplantation [4–6]. The literature reports a 65 to 250-fold increase of SCC occurrence in OTRs. The ratio of SCC to basal cell carcinoma (BCC) in OTRs changes from 0.2 : 1 in the general population to 2.7 : 1 in OTRs with long duration of immunosuppression [1, 6–9]. These ratios differ in several publications which can be caused by dissimilar groups of patients and factors taken under consideration. The course, clinical and histopathological features of SCC in OTRs are different compared to immunocompetent patients, so it is crucial to bring the significance of this fact to the attention of clinicians. In this population SCC has a more aggressive course, metastasizes more frequently and tends to have multifocal growth.
Factors of unfavourable prognosis of SCC in OTRs are multiple. They include factors shared with immunocompetent patients, such as older age, skin phototype, location on a head/neck, tumour size, multiplicity of lesions, higher UV exposure, specific histological features, association with scars, burns, chronic wounds, presence of extracutaneous tumours and HPV/bacterial infections. Transplantation related factors include mainly the immunosuppressive treatment, but also duration of dialysis before transplantation, drug intake during pre-transplantation period, type of transplanted organ, cause of organ insufficiency and occurrence of NMSC tumour before transplantation [10].
Clinical SCC features
Most SCC foci are formed as a consequence of precancerous lesions in both organ recipients and the populations of immunocompetent patients. SCCs are most frequently localised in the areas that are exposed to solar radiation, whereas the foci of BCC most frequently develop on the back and chest.
Similarly to solar keratosis, the SCC localisation depends on age and is similar in both populations. Before the age of 40, most lesions are located on the back of the hands, forearms and the torso, whereas after the age of 40 the head is the dominant location [11]. In males, the SCC most commonly occurs on the head and neck, whereas the most popular locations in females are on the torso. The SCC foci in women are only occasionally located on ears and the scalp, which is probably linked to the protective role of hair [4, 12].
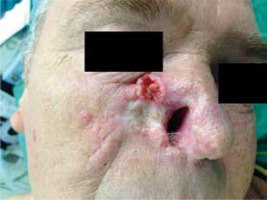
Among the general population, the SCC may assume two clinical forms: an exophytic tumour or a penetrating ulcer (Figure 1). SCC foci in immunocompetent patients are usually characterised by a slow growth, lack of pain, infiltration, bleeding erosions, necrotic ulcerations, or assume the form of nodules or tumours [4, 13]. In clinical terms the picture of SCC in OTRs is quite similar to the populations of immunocompetent patients. However, in OTRs SCC more frequently manifests as a series of fast growing multifocal tumours which are potentially life threatening. In both populations (OTRs and general) SCC may take the form of a flat protruding erythematous focus with surface peeling which may initially be mistaken for a chronic inflammation. A fast, progressive growth of the neoplasm causes the foci to become lumpy, with a frequently observed disintegration on top. A penetrating ulcer covered with a scab is yet another clinical presentation, which happened in first of the described patients with ulceration penetrating into soft tissues. SCC foci are most commonly connected with numerous viral warts and the foci of solar keratosis. SCC tumours are usually accompanied by symptoms of skin damage caused by solar radiation, such as elastosis, pigmentation disorders and telangiectasia. In the case of post-transplantation patients where the age at which the SCC occurs is normally lower than in immunocompetent patients, fewer indications of solar skin damage are observed. It is important to note that slight SCC in OTRs may imitate benign lesions and may delay correct diagnosis [4, 13–15].
Figure 1
SCC with an expansive ulceration on the right cheek and nose in a patient after renal transplantation who developed few recurrences of the tumour
In OTRs, one distinctive factor is the painfulness of SCC foci, which may indicate an invasive SCC form. Bouwes Bavinck et al. conducted a trial on 410 patients from the post-transplantation population, which proved that feeling pain within the lesion may be an independent factor confirming the occurrence of an invasive SCC form. Further observations that Oh et al. conducted on the same group of OTRs provide evidence that developing painful lesions may predict an increased overall mortality risk. There was also a positive association between the number of painful lesions and overall mortality. This important symptom helps to encourage patients to seek treatment earlier [16, 17].
It is noticeable that skin cancer in post-transplantation patients has a tendency to a multifocal growth pattern and co-occurrence. Most of the literature data point to the fact that skin cancer in post-transplantation patients is in 50% of cases multifocal. In such cases the first and the second recognised neoplasms were predominantly BCCs, followed by SCC [9, 18].
It is important to stress the differences between publications comparing the frequencies of BCC and SCC among OTRs. Sułowicz et al. in their study on 468 patients after KTx presented that the ratio of BCC : SCC was 2.79 while the ratio of SCC : BCC equalled 0.36 for the entire population of patients after KTx [19]. The first diagnosis of NMSC occurred 2–170 months after the transplant surgery and this time did not differ significantly between BCC and SCC (p = 0.7). A Slovak publication by Zilinska et al. also shows that amid 1421 patients, who underwent renal transplantation, the most frequent malignancies were skin cancers: BCC in 17.6% and SCC in 8.2% [20]. As the authors suggest, the type of malignancy is different in various countries and dependent on genetic and environmental factors [21]. A population-based study run in Sweden describes a higher risk of BCC than SCC in the first years of observation [22]. The authors stress that the low SCC to BCC ratio was possibly attributed to short follow-up time. The same conclusions were shown in a retrospective report made by Zavos et al. [23]. In a group of 1736 patients who underwent renal transplantation between 1983 and 2008, 2.2% were diagnosed with NMSC (SCC : BCC ratio was 3.6 : 1). Interestingly, the mean time to post-transplant presentation was shorter in BCC (62.1 months) than in SCC (103 months), which strengthens previous assumptions even more. It must be highlighted that SCC in transplanted patients have a tendency to appear at a younger age when compared to immunocompetent people and they metastasise more often. Most metastases appear up to 15 months after the removal of the primary focus, but later lesions have been recorded too [4, 9].
In the case of the SCC, different clinical presentations are observed. The most frequently occurring presentations include: keratoacanthoma, Bowen’s disease, spindle-cell squamous carcinoma and verrucous carcinoma.
Histopathological features
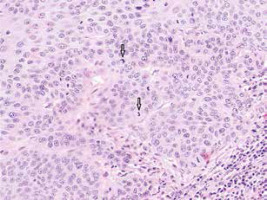
In most cases the characteristic features of the histopathological presentation of SCCs in OTRs are similar to those observed in immunocompetent patients. In the cancer foci a proliferation of atypical keratinocytes with varying degrees of differentiation and keratosis has been observed (Figure 2). In the nuclei of neoplastic cells, numerous figures of division are present, including atypical figures. In highly differentiated cancers one observes a formation of oval structures built from concentrically positioned keratinizing cancer cells and concentric layers of keratohyalin called keratin pearls. Inflammatory infiltration is composed of lymphoid cells, plasma cells, eosinophil granulocytes, and features of dermal fibrosis present in the stroma indicate an intense immunological reaction of the host organism [13, 24].
Figure 2
Moderately differentiated SCC with numerous mitotic figures (arrows) and tumour cells with marked keratinisation
The literature reveals that the number of cells with a higher degree of atypia is greater in OTRs compared to the general population. However, new research has proved there is no increased rate of poorly differentiated SCC in OTRs as compared to the general population. Moreover, in immunosuppressed patients the SCC tumours are more often characterised by the presence of multinucleated giant cells [25, 26]. The squamous cell carcinoma cells demonstrate a positive immunohistochemical reaction with the use of antibodies against cytokeratins, whereas in tumours with a low degree of differentiation, with antibodies against vimentin. An exceptionally rare pseudovascular variety of the squamous cell carcinoma in the general population is much more common among OTRs [27]. The inflammation formed around the foci of the SCC in OTRs is less intense, which may be responsible for the tumour aggressiveness [28]. Furthermore, the Krynitz et al. experiment demonstrated that there are differences in the cell composition of the infiltration around the SCC in patients subjected to immunosuppression, which consequently may lead to a quicker progression of the tumour in this population. In the OTRs it was found that the number of lymphocytes T (CD3+) and monocytes was smaller, whereas the number of plasm cells was increased. The density of the cell distribution was similar in both groups, which suggests disturbances in the functioning of the cells [29].
Among OTRs and immunocompetent patients who developed a SCC with a viral aetiology, koilocytosis was observed with the same frequency. Simultaneously, in another experiment it was observed that koilocytosis in connection with at least one other feature that suggested the HPV infection, i.e. symmetry of the lesion and its verrucous structure, growth of the granular layer, hyperkeratosis, parakeratosis, swollen dermal capillaries, was observed more often in post-transplantation patients [28].
Course and prognosis
An increased activity and invasiveness of SCC in OTRs compared to the immunocompetent population is quite well documented. There are still insufficient data in the literature about the destructive and/or fatal course of SCC among OTRs. Lott et al. conducted a study to evaluate factors of aggressiveness of nonmelanotic skin cancers (NMSC) in OTRs compared to the immunocompetent population. In 7021 patients after transplantation, 153 had documented SCCs. Deep tissue involvement or perineural invasion occurred in 25 patients, while in the immunocompetent group only 4 patients had these features. 3 patients died from SCC in OTR group comparing to 1 in the control group [30].
In OTRs there is also an increased risk of multifocal tumours as well as the occurrence of metastases. Neoplasms in OTRs grow faster, are bigger and more malignant when compared to those occurring in the general population.
Metastasis can be the principal cause of death in post-transplantation patients. It is described that SCC has an increased tendency for local recurrences in around 13% of adult OTRs, usually in the course of the first 6 months after the resection of the lesion. The risk of SCC metastasis in organ recipients is about 7% within 2 years from the resection of the tumour, whereas a general population risk of metastasis is about 2% [31].
The risk factors for the aggressive course, relapse and SCC metastases in OTRs include: old age, excessive sun exposure, multifocal SCC, tumour size larger than 2 cm, indistinct lesion boundaries, rapid growth, ulceration within the tumour, presence of extracutaneous tumours, as well as lesions located in the central part of the face, eyelids, eyebrows, near ears and eyes, nose, mouth, chin, jaw, temple, genitals and fingers [4, 32].
The histological features which testify to the aggressiveness of the tumour are low differentiation, thickness of over 5 mm, and infiltration of the tissues located below (subcutaneous tissue, nerves, cartilage, muscles and bones). The prognosis for patients with metastasis is poor: death rates vary from 13% to 46% over 2 to 4 years. The research by Martinez et al. revealed that the average time of the first metastasis diagnosis was 10.7 years after transplantation. It was also demonstrated that the metastases developed soon after the appearance of the primary neoplasm focus, within 1.4 years on average [33, 34].
Neoplastic cells in OTRs metastasise chiefly to regional lymph nodes, similar to the populations of immunocompetent patients. For this reason lymph nodes should be routinely examined in all SCC patients during assessment. The lymph nodes that are most commonly at risk in both OTRs and the general population are located in the head and neck area [4].
The skin that surrounds the primary neoplastic focus needs to be thoroughly checked for satellite tumour nodules. In-transit metastatic spread occurs considerably more frequently (about 26%) in the case of high-risk SCC tumours in organ recipients as compared with the general population. The prognosis for patients with such metastases is unfavourable; the mortality rate within 2 years of appearance is about 33%. Chronic immunosuppression exacerbates the prognosis for patients with metastatic spread [35].
Metastasis to internal organs is less frequent but the prognosis is very poor. SCC in OTRs most frequently metastasises to lungs and bones. The survival rate in organ recipients with metastases is 56% after 3 years, and 34% after 5 years, compared to the general population where, surprisingly, it is significantly lower at 25% after 5 years [36].
Patients with either in-transit metastases, or metastases to regional lymph nodes have more positive prognoses than the patients with metastases to distant lymph nodes or internal organs [35]. Certain skin cancer metastases to regional lymph nodes are potentially curable, which underlines the importance of their thorough examination during every check-up (physical examination, ultrasound assessment).
The summary of the main differences between SCCs in OTRs and immunocompetent patients is presented in Table 1.
Table 1
Summary of main differences between SCCs in OTRs and immunocompetent patients
Treatment
Surgical resection, including Mohs micrographic surgery, wide local excision, electrodessication and curettage of the lesion, is the therapy of choice and sufficient in the majority of SCC cases in both populations. With lesions spreading superficially and cancers in situ, treatment also includes cryotherapy and local topical treatment with 5-fluorouracil and imiquimod, as well as with ingenol mebutate. Typically, systemic treatment is not required in the immunocompetent population.
OTRs are more often in a group which develops “high-risk” cancers, those tumours are recommended to be managed by a multidisciplinary treatment team with treatment algorithms including complete skin and regional lymph nodes examination, imaging as appropriate, excision with wider margins or Mohs micrographic surgery with margin assessment, and adjuvant therapies as appropriate.
Adjuvant therapies are still a subject for research and clinical trials. Acitretin is a systemic retinoid used as adjuvant therapy in OTRs for NMSC reduction and is often considered in patients with a significant SCC burden, typically between 5 and 10 SCCs a year. Retinoids are thought to reduce NMSC by promotion of cell differentiation, growth regulation in hyperproliferative epithelia and downregulation of proto-oncogenes by binding nuclear receptors [37].
In the population of OTRs latest research shows high efficacy in treatment of SCC with capecitabine as a chemoprevention and adjuvant therapy as well. Capecitabine is a prodrug of 5′-deoxy-5-fluorouridine that is converted enzymatically to its active metabolite 5-FU. It was initially approved by the US Food and Drug Administration for breast cancer and subsequently for metastatic and primary colon cancer. Endrizzi et al. achieved a mean reduction in monthly SCCs of 61.8 ±29.8% after 12 months of capecitabine treatment and 53.4 ±43.1% after 24 months of treatment in the OTR population [38, 39].
Radiotherapy is also being considered as adjuvant therapy in case of the aggressive course of SCC, large perineural invasion, coexisting regional metastasis or a high risk of regional metastasis. It is noticeable that immunosuppressed patients tend to have decreased response rates compared to those with an intact immune system.
Another therapy method that is still under discussion among SOTRs is immunotherapy with immune checkpoint inhibitors (ICI). Cemiplimab, the anti-programmed cell death protein (PD) 1, is the only ICI approved for SCC, followed by pembrolizumab, the PD1-inhibitor as a second line treatment [40, 41]. A challenge of great importance is balancing between reduced efficacy of ICI because of immunosuppression and potential risk of allograft rejection. A shorter period of time between transplantation and onset of ICI is said to be a risk factor for organ failure [42]. Studies show that modification of immunosuppression therapy as reduction of calcineurin inhibitor, conversion of mycophenolate to mTORi and increased doses of steroids may have a role in prevention of allograft rejection in SOTRs under ICI treatment [43].
It is important to adhere to advice on prevention of these neoplasms in both populations, however in post-transplantation patients prevention should be more extensive and involve the use of protective wear in summer months (long-sleeved shirt, a wide-brimmed hat). Fabrics that have the best ultraviolet protection factor (UPF) are tightly woven fabrics, dark colours, wool and polyester.
Moreover, the use of sunscreen with sun protection factor (SPF) of 50+ should be advised. Caution is advised when using physical filters on traumatized skin as no safety data exist for this application.
Supplementation with specific antioxidants, such as β-carotene, selenium, vitamin A, E and C is not recommended. Randomized control trials did not reveal any benefit, and in some cases the risk of NMSC was even increased [44].
Conclusions
SCC is the most frequently occurring skin cancer in post-transplantation patients. The frequency of SCC occurrence in organ recipients increases by 65–250-fold. In the population of post-transplantation patients the SCC foci have a more differentiated clinical picture, the rate of appearance of the tumour progression may be much quicker and the course of disease much more aggressive. The risk of local relapse and the metastases has been reported considerably more often than in immunocompetent persons. Therefore, these neoplasms may cause both diagnostic and therapeutic problems.
Because OTRs are at increased risk of developing and dying from both nonmelanoma and melanoma skin cancers, annual screening for skin cancer including clinical skin examination is recommended for all OTRs and should be performed by a dermatologist. Whereas a regular clinical skin examination is not recommended for the general population, and the evidence of survival benefit of population-based screening is limited and restricted to the detection of early stage melanoma in certain populations [45].
On account of the frequent occurrence of skin cancers in post-transplantation patients, it is essential that regular dermatological examinations are conducted in the OTR population and information on possible prevention is provided.








