Introduction
Asthma is an important pulmonary disease that threatens public health in all countries, and affects more than 350 million people. Both genetic predisposition and environmental factors contribute to the development and exacerbation of asthma [1–3]. In asthma, aetiology is not fully understood, but its pathogenesis is involved with multiple immune cells and related signalling pathways such as T helper 2 (Th2) pathway and eosinophilic inflammation. Excessive reactive oxygen species (ROS) production plays a main role in the asthma pathogenesis, and recent studies implied that GATA3 (main gene of Th2), MUC5AC (main gene of mucus production), and KIAA1109 have an association with the moderate-to-severe asthma development susceptibility. The MUC5AC has an important role in airway remodelling and tethering impairs mucociliary transport, which is likely to be main contributors to obstruction of the airway and asthma mortality [4–6].
Aluminium Oxide Nanoparticle (alpha-alumina, α-Al2O3, 99.9%, 50 nm) is thermodynamically stable and important material for therapy applications [7]. The mucus-binding ability of Lactobacillus fermentum is mediated by a mucus adhesion promoting protein (MapA), which is known as a cell-surface protein [8, 9].
Vasoactive intestinal peptide (VIP) consists of 28 amino acid residues and is distributed in the immune and nervous systems. Abundantly, VIP is present in the lung and VIP-immunoreactive nerves are involved in the glands and smooth muscle layer of airways, and walls of pulmonary vessels. VIP exerts wide biological functions, such as neuromodulation, smooth muscle relaxation, anti-inflammation and immunomodulation that are mediated via interaction with two subtype receptors VPAC1 and VPAC2. VIP acts as a potent bronchodilator and VIP deficiency is a pathogenic factor in asthma. It was also presented that VIP enhances phosphatidylcholine synthesis, the major component of surfactants in the pulmonary system, by enhancing choline-phosphate cytidylyltransferase (CCT) and CCTα mRNA via its receptor-mediated pathway. Herein, in systemic administration, the rapid degradation of VIP is the main limitation of its clinical applications as a therapeutic agent. In addition, systemic administration of VIP can cause side effects in the cardiovascular system [7, 10].
About asthma, more of the recently developed medications had no ability to control all aspects of asthma pathophysiology. Based upon recent findings, administration of exogenous VIP may be effective for the treatment of asthma in clinically practice [7, 11]. Therefore, the effective drug delivery system to the target tissue is necessary and for lung administration, developing the formulation for pulmonary administration could be applicable and using VIP with delivery system and target binding molecule (MapA to the mucus of the airway) would be applicable in anti-asthma-therapy.
Material and methods
VIP-MapA preparation
Preparation of VIP-MapA attachment to alpha-alumina was done according to the previous study [7] and in brief, after preparation of a-alumina, VIP and MapA were attached to alpha-alumina particles.
Treatment schedule
BALB/c mice were kept under standard laboratory conditions and were divided in 4 groups (n = 7 mice). To produce an allergic asthma mouse model, ovalbumin (OVA) was used for sensitization and challenge according to a previously studied protocol [12, 13]. Four groups include: healthy control group (PBS group) that was sensitized and challenged by PBS, allergic asthma mouse model group (OVA group), allergic asthma mouse model group that was treated with VIP (VIP group) and allergic asthma mouse model group that was treated with VIP-MapA-α-alumina (VIP-MapA group), on days 25 and 29. On day 31, mice were euthanized and sampling was done.
BALF cells
Broncho-alveolar lavage fluid (BALF) was collected from lungs. After preparation and staining of produced slides, the percentage of eosinophils was determined.
IgE level
The immunoglobulin E level (total IgE) was measured in blood serum samples (that were taken on day 31) with a specific ELISA kit.
Cytokines level measurement
BALF was centrifuged and supernatants were applied to determine the levels of cytokines (IL-4, IL-5, and IL-13) by using specific ELISA kits.
Gene expression
For study expression of two genes GATA3 and MUC5AC, the cells in the BALF were isolated, and from these cells, total RNA was extracted. After synthesis of cDNA, quantitative real time PCR was applied and the GAPDH gene was used as an internal gene.
Histopathology
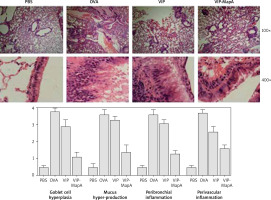
Lung tissues of mice were isolated and fixed. Then slides were stained with hematoxylin and eosin (H&E), and evaluated by light microscopy to survey mucus hyper-production, goblet cell hyperplasia and eosinophilic inflammation in the perivascular and peribronchial area.
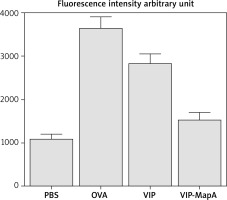
ROS determination in BALF
For measurement of ROS in BALF, BALF cells were washed and DCFDA was incubated in DMSO. The microplate fluorescence reader was used to measure fluorescence intensity at excitation and ROS level was expressed in arbitrary units.
Results
BALF cells
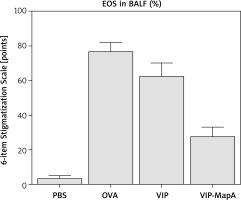
Eosinophils as main cells in airways of asthma were increased in BALF of the OVA group (77%) compared with the PBS group (4%) (Figure 1). Administration of VIP and VIP-MapA as treatment could decrease eosinophils (63% and 28%, respectively) in BALF.
IgE level
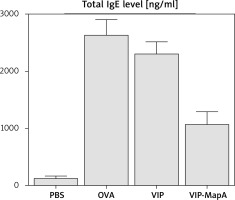
The total IgE level in serum was increased in the OVA group (2639 ng/ml) in comparison to the PBS group (133 ng/ml) (Figure 2). Administration of VIP and VIP-MapA as treatment could decrease the total IgE level in serum (2312 and 1083 ng/ml, respectively).
Cytokine levels
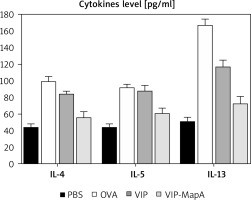
The levels of three main allergic asthma-related Th2 cytokines (IL-4, IL-5 and IL-13) were studied; they were increased in the OVA group (99, 92, and 167 pg/ml, respectively) as compared to the PBS group (44, 43, and 51 pg/ml, respectively) (Figure 3). Administration of VIP and VIP-MapA as treatment could decrease IL-4, IL-5 and IL-13 in BALF.
Gene expression
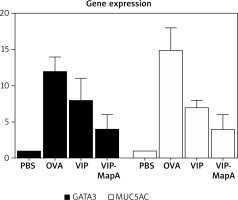
The gene expression of GATA3 and MUC5AC were enhanced in the OVA group (12 and 15 relatively expression, respectively) compared to the PBS group (1 and 1 relatively expression, respectively) (Figure 4). Administration of VIP and VIP-MapA as treatment could decrease GATA3 (8 and 4 relatively expression, respectively) and MUC5AC (7 and 4 relatively expression, respectively) gene expression.
Histopathology
In the OVA group, perivascular and peribronchial eosinophilic inflammation and also, hyperplasia of the goblet cell and mucus hyper-production were increased in comparison to the PBS group (Figure 5). Administration of VIP and VIP-MapA as treatment could decrease perivascular and peribronchial eosinophilic inflammation, hyperplasia of the goblet cell and mucus hyper-production.
ROS level
ROS level was increased in the OVA group and treatment with VIP and VIP-MapA could ameliorate an increased ROS level (Figure 6). Both treatment strategies were effective in reduction of the ROS level compared to the non-treated OVA group.
Discussion
Bronchial inflammation via eosinophil infiltration with mucus hyper realizing leads to airway obstruction that is the main problem in asthma and controlling of this problem is the main therapeutic goal in asthma. In current clinical practices, the eosinophil and IgE level, as markers of eosinophilic inflammation, are measured in type 2 allergic asthma [14, 15]. In our study, treatment of asthmatic mice with VIP-MapA could control perivascular and peribronchial inflammation that were mainly eosinophilic inflammation and also, goblet cell hyperplasia and mucus hyper-production, which are main factors in bronchial obstruction. This effect was stronger in the VIP-MapA than in the VIP group that showed an enhancing effect of MapA on the stability of VIP.
VIP has a wide range of biological functions and can act as a neuromodulator of the inhibitory non-adrenergic and non-cholinergic nervous system of airways, which influences many aspects of pulmonary biology. VIP deficiency is a causative factor in the respiratory disease pathogenesis. In the clinical application, VIP was offered as potential treatment for chronic inflammatory pulmonary diseases such as asthma; however, its application has several limitations, including difficulty in administration routes, extremely short half-life in plasma after intravenous administration, and its dangerous side effects on the cardiovascular system [16]. VIP plays an important role in the neuroendocrine–immune response and participates in cell differentiation, and smooth muscle relaxation as well as VIP inhibits promotion of Th1 to Th2 transformation. Proliferation and hypertrophy of airway smooth muscle cells (ASMCs) are responsible for airway remodelling that leads to irreversible pathological changes in the airway. Caveolin (Cav)-1 on the surface of ASMCs is involved in cell cycle and signal transduction regulation, which allows ASMCs to change from proliferation to apoptosis. The signalling pathway of the extracellular signal-regulated kinase (ERK)1/2 is a common pathway regulated by several proliferative factors, which demonstrates a regulatory role in airway remodelling of asthma. The effect of VIP on IL-13-induced ASMCs proliferation was studied via the VIP effects on phospho-ERK1/2, ERK1/2, and Cav-1 expression in ASMCs. VIP inhibits the ERK1/2 signalling pathway phosphorylation and Cav-1 expression on ASMCs, thus inhibits proliferation of the ASMCs [17]. The GATA3 is the main regulatory and differentiation gene of Th2 and MUC5AC is the main gene in mucus production. These two genes were overexpressed in allergic asthma and led to Th2 immune response dominant and mucus production, respectively, that are main factors in asthma pathogenesis. Administration of VIP-MapA as asthma treatment could decrease GATA3 and MUC5AC relative expression.
VIP immunoreactivity was observed in all lymphoid organs and is evidence for the synthesis of VIP by immunocytes. VIP regulates biological activities through its receptors, which are positively coupled to adenylyl cyclase. Also, VIP can be a physiological cytoprotective molecule in pathophysiological situations in which immunocompetent cells are involved [18]. VIP, as an immunomodulator and anti-inflammatory agent, acts through its specific receptors: VPAC1 and 2. Stabilized VIP derivative (IK312532) was suggested as an anti-asthma drug candidate and was developed for inhalation therapy [19]. It was observed that in this study VIP-MapA acted as a strong immunomodulator and this effect was more powerful than for VIP alone. VIP-MapA could reduce main Th2 and allergic asthma related cytokines: IL-4, IL-5 and IL-13 that were increased in the OVA group.
It was shown that the inhaled VIP is rapidly degraded by endogenous proteases and intravenous administration of VIP has a cardiovascular side effect. VIP in the circulation, as well as other natural peptides, is subject to the rapid chemical and enzymatic degradation after the systemic administration. To overcome such shortcoming of VIP, a carrier to prevent the self-aggregation and, maintaining a certain functional form of VIP is necessary. Using erythritol as a carrier, with high stability, was recently reported for intratracheal administration, VIP binds to VIP receptors in lung. Thus, manipulated VIP is advantageous for the therapy of lung diseases and a pharmacologically useful drug for asthma [20]. The stable VIP derivative for inhalation therapy had no degradation and conformational changes and exhibited high inhalation performance. It attenuated inflammatory symptoms in experimental asthma model rats and decreased infiltrated granulocytes [21]. In the current study, manipulated VIP is more advantageous than VIP alone for the therapy of asthma. The total IgE level as a main laboratory marker of allergic diseases was significantly reduced by VIP-MapA treatment in comparison to VIP. Also, the ROS level was decreased in the VIP-MapA group and VIP-MapA could ameliorate the increased ROS level more than VIP.
In a study of 2004, the effect of synthetic VIP-like analogues (the pituitary adenylate cyclase-activating peptide or PACAP) on cytokine induction in airways was evaluated. Systemic administration of the PACAP analogue attenuated the cytokine-induction in BALF and also, local administration of the VIP analogue inhibits cytokine-induced neutrophil recruitment in airways [22]. Eosinophils as main cells in airways of allergic asthma, were decreased in BALF with administration of VIP-MapA and this effect was more highlighted in VIP-MapA than in VIP.
The mucus layer is a complex arrangement of mucin glycoproteins, which plays a main role in protecting the epithelial surface from harmful foreign substances and pathogens. The mucus layer is periodically cleared; any entrapped materials are removed for excretion. Muco-adhesive systems play an anchor-like role at the site of mucus and have strong interactions with mucus to prolong residence; as such, these systems are attractive candidates for long-term drug delivery. However, one major drawback of muco-adhesive systems is that they cannot access the underlying epithelium, which could influence possible applications and it will have difficulty in penetrating the mucus layer [23]. Lactobacillus strain, a member of lactic acid bacteria, is characterized in fermentation of food and can enhance the intestinal barrier function as the probiotic strain. Lactobacillus adheres and aggregates in the gastrointestinal tract to promote maturity of biofilm and the extracellular matrix secreting through the signal molecules in the quorum sensing system [24]. Therefore, adhesion properties of cell surface proteins derived from Lactobacillus strains provides a valuable vehicle to target mucus containing tissues.
Lactobacillus adhesion can be done with a mucus-producing goblet cell and bacterial cells adhere to the mucus [25, 26]. The mucus-binding protein has a role in the adhesion and immunomodulatory activity of Lactobacillus. Furthermore, mucus-binding protein triggers immune regulation through the Toll-like receptor 4 (TLR4) signalling pathway and inhibits the activation of mitogen-activated protein kinase (MAPK) signalling pathway [27]. VIP has an immunomodulatory effect on the control of asthma but it cannot be used alone because it has many side effects and this peptide can be eliminated or destroyed. We used a new delivery system to carry VIP in the lung via inhalation and with use of MapA as an adhesion molecule, and VIP could stay on mucus of airways. Therefore, MapA acted as an anchor and VIP could reach the airway. On the other hand, use of alpha-alumina led to easy carrying of VIP and was a linker between VIP and its anchor (MapA). Also, it could increase density of VIP-MapA and led to penetration of this component to the mucus and reach bronchial cells, which presents an effective, strong, long-acting and applicable asthma therapy.