Purpose
Basal cell carcinomas (BCC) are the most common of all skin cancers, accounting for about 80% of non-melanoma skin cancers (NMSC). The vast majority of BCCs, as much as 80-85%, are located in the head and neck (H&N) region that is most exposed to oncogenic effects of ultraviolet radiation [1-4].
According to the National Comprehensive Cancer Network (NCCN), the most important and clinically helpful division of BCC is the one into tumors with a low- and high-risk of recurrence (RR) [5]. The most significant factors increasing the risk of BCC recurrence within four years after treatment include the location of lesion in the H&N region, and the fact that it is a recurrent lesion after previous therapy. Both features qualify the tumor to the high-RR group (HRG) [5-7].
Surgery is currently recognized as the primary treatment method for BCC. Mohs micrographic surgery (MMS) is a surgical technique recommended in the first place by NCCN in BCC from HRG. According to two meta-analyses published in 1989 by Rowe et al., the number of local recurrences after five years from MMS for primary and recurrent BCC was only 1% and 5.6%, respectively, proving high effectiveness despite worse outcomes in the treatment of relapses [8, 9]. However, the MMS procedure is difficult, time-consuming, and costly [10]. This is probably why it is not covered by health insurance in many countries outside the USA [11]. Consequently, the most common method for HRG-BCC treatment in our country is standard surgical excision (SSE) with post-operative margin assessment (POMA). However, according to a randomized clinical trial comparing the treatment efficacy in recurrent BCC of the facial skin, the 10-year recurrence rates reach 13.5% after SSE with POMA, and only 3.9% after MMS (p = 0.023) [12].
The NCCN also recommends radiation therapy in HRG-BCC, but only when radical surgery is impossible, or the patient does not consent to it. At the same time, the recommendation argues that when preservation of function and cosmetic effect are of concern, especially in the H&N region, radiation therapy should be considered as the method of the first choice [5].
Unfortunately, the vast majority of retrospective studies describing treatment with external-beam radiotherapy (EBRT) or brachytherapy (BT) concern heterogeneous groups of patients diagnosed with both squamous cell carcinoma (SCC) and BCC. A meta-analysis of these studies by Zaorsky et al. showed that the effectiveness in terms of 1-year local control was above 93% for both methods, with a slight predominance of better cosmetic effects for BT [13].
Nonetheless, the collective presentation of treatment results on two completely different biological neoplasms, such as SCC and BCC, causes a lack of transparency. Especially in the field of radiotherapy, where the higher radiosensitivity of BCC compared with SCC was already proven in the 1980s [14, 15].
The authors of this study were not able to identify any scientific reports on the results of treating only BCC of the H&N region by high-dose-rate brachytherapy (HDR-BT), both in case of primary lesions and relapses after previous SSE. Therefore, the purpose of this manuscript was to fill this gap in the literature.
Material and methods
The retrospective study was based on patients’ data obtained from electronic medical records. The study was not considered a medical experiment. It did not require an approval of the Bioethics Committee of the Poznan University of Medical Sciences, which was confirmed by the decision of the Chairman.
Medical histories of 510 patients with various skin cancers treated using multiple techniques and brachytherapy regimens from March 2012 to February 2017 at the Brachytherapy Department of the Greater Poland Cancer Centre in Poznań were analyzed. Four patients’ inclusion criteria were established for further investigation: histopathologically confirmed BCC, H&N area of tumor localization, radical treatment performed with HDR-BT in two-dimensional (2D) planning with 10 × 5 Gy fractionation scheme, minimum 12 months of follow-up from the end of treatment.
Superficial 2D HDR-BT procedure
Only applicators of the Freiburg flap type were used, which were attached directly to the skin of patients with medical patches. Planning was done by covering the lesion with a margin of at least 5 mm with an applicator. HDR MicroSelectron v.3 (Nucletron, ELEKTA Company, Sweden), containing iridium-192 (192Ir) with a nominal activity of 10 Ci was used for treatment. Reference isodose was prescribed 5 mm below the surface of the skin. 2D planning was performed with Plato or Oncentra-Brachy software (Nucletron, ELEKTA Company).
Fractionation
The planned physical dose was 50 Gy in 10 workdays fractions, and biologically effective doses (BED) were estimated for both BCC cells, and for skin early reactions (α/β = 10 Gy) and late skin reactions (α/β = 3 Gy). Linear-quadratic model-based formula was applied for calculations: BED = nd [1 + d/(α/β)], where n is the number of fractions, d is the fractional dose, α is the coefficient determining cell death because of the passage of one radiation quantum, β is the coefficient determining cell death because of the passage of many radiation quanta [16, 17].
Follow-up
On the last day of HDR-BT, all patients were advised to use a prescription ointment with neomycin and vitamin A in the event of acute radiation reaction in the form of wet exfoliation. Early radiation skin reactions were assessed at the first control visit, 4-5 weeks after HDR-BT. During subsequent examinations performed at 2-6 months intervals, local control (LC) and late radiation reactions were evaluated. Treatment toxicity was rated according to criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) scale [18].
Data collection and statistical analysis
Statistical analyzes were performed with Statistica (data analysis software system), version 12 (TIBCO Software Inc.). In one case, it was necessary to use Fisher-Freeman-Halton test, which was carried out using Cytel Studio version 9.0.0 (Cytel Inc.). The level of statistical significance was established with p-value < 0.05.
Results
Based on the adopted criteria, 90 patients with 102 tumors were qualified for the study. Patients and tumors were divided into two sub-groups, including the primary treatment group (PrG) and the recurrent treatment group (ReG) treated for local recurrence after previous SSE. Two patients were included in both the groups due to simultaneous treatment of primary and secondary lesions.
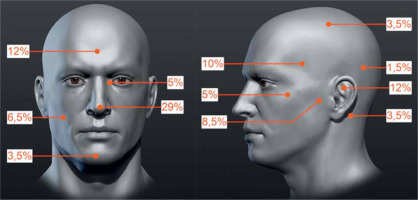
The PrG included 59 tumors in 50 patients, with a mean follow-up of 43.7 months. Of these, 43 patients were treated with one tumor, in 2 patients, three tumors were treated simultaneously, and in the remaining 5 cases, two tumors were treated. The group consisted of 24 women and 26 men, in whom 25 and 34 neoplastic lesions were treated, respectively. The mean patients’ age was 72.8 years (median, 72.9 years; range, 47.8-89.9 years). The tumors were classified according to TNM for the scalp and neck skin cancers [19] as T1 in 47 (79.7%) and T2 in 12 (20.3%) cases. Due to the size and location, 100% tumors were rated as HRG, according to NCCN. The percentage distribution of tumors at precise anatomical locations is shown in Figure 1.
Fig. 1
Percentage distribution of tumors primarily treated with high-dose-rate brachytherapy (HDR-BT). Specified areas: temporal, parietal, frontal, occipital, auricle, preauricular, retro-auricular, buccal, zygomatic, chin, nose, and the inner eye canthus (the figure uses anatomical model created by Stefan Polster, available at https://www.artstation.com/artwork/X28Yl)
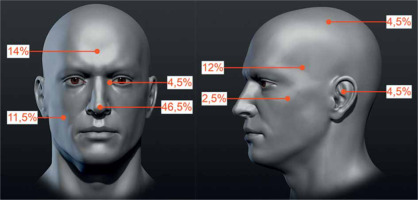
The ReG included 43 tumors in 42 patients, with a mean follow-up of 43.5 months. Forty-one patients were treated with one tumor, while one patient with two tumors. The group consisted of 24 women and 18 men, in whom 24 and 19 neoplastic lesions were treated, respectively. The mean patients’ age was 70 years (median, 72.9 years; range, 32.9-89.9 years). The tumors’ local clinical advancement according to the same TNM classification was T1 in 35 (81.4%) and T2 in 8 (18.6%) cases. As all were recurrent, 100% lesions were classified into HRG according to NCCN. The percentage distribution of tumors at exact anatomical locations is shown in Figure 2.
Fig. 2
Percentage distribution of high-dose-rate brachytherapy (HDR-BT)-treated tumors due to local recurrence after prior surgical treatment. Specified areas: temporal, parietal, frontal, auricle, buccal, zygomatic area, nose, and the inner eye canthus (the figure uses anatomical model created by Stefan Polster, available at https://www.artstation.com/artwork/X28Yl)
The calculated BED for tumors and early reactions was 75 Gy, while for late reactions, it was 133.3 Gy, and was the same in both the groups. Both the groups’ parameters were subjected to comparative statistical analysis. No statistically significant differences were found between the groups, which indicates their high similarity in the scope of presented data. The results are summarized in Table 1.
Table 1
Comparative statistical analysis of the studied groups
| Variable | Primary treatment group | Recurrent treatment group after SSE | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| n | mean ±SD | median | n | mean ±SD | median | |||
| Age (years) | 59 | 72.8 ±9.1 | 72.9 | 43 | 70.0 ±12.5 | 72.9 | 0.43* | |
| OTT (days) | 59 | 11.25 ±1.09 | 11.0 | 43 | 11.05 ±0.62 | 11.0 | 0.17* | |
| Follow-up (months) | 59 | 43.7 ±15.3 | 41.4 | 43 | 43.5 ±16.9 | 43.5 | 0.96* | |
| Sex | ||||||||
| Female | 25 | 24 | 0.18** | |||||
| Male | 34 | 19 | ||||||
| TNM | ||||||||
| T1 | 47 (79.7%) | 35 (81.4%) | 0.83** | |||||
| T2 | 12 (20.3%) | 8 (18.6%) | ||||||
| Localization | Showed in Figure 1 | Showed in Figure 2 | 0.34** | |||||
HDR-BT effectiveness
In the PrG, Kaplan-Meier estimated 3- and 5-year relapse-free survival (RFS) was 96.4% (Figure 3). There were only two local recurrences (LRs) in two patients treated for single lesions (2/59, 3.39%). The first occurred 12 months after the treatment of T1 tumor of the auricle, and the second appeared 24 months after the treatment of T2 tumor of the preauricular region.
Fig. 3
Graph showing a comparison of Kaplan-Meier curves of relapse-free survival in the primary treatment group (PrG) and the recurrent treatment group (ReG)
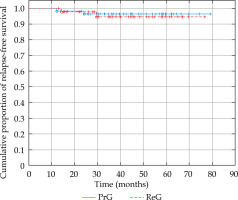
In the ReG, Kaplan-Meier estimated 3- and 5-year RFS was 94.6% (Figure 3). This group also experienced two LRs (2/43, 4.65%). The first occurred 14 months after the treatment of T1 tumor of the tip of the nose, and the second appeared 29.5 months after the treatment of T1 tumor of the auricle.
The log-rank test was applied for statistical comparison of RFS in both the groups, and no statistically significant difference was found in the number of recurrences (p = 0.72, Figure 3).
HDR-BT toxicity
In the PrG, early toxicity was reported in all treated areas during the first control visits. In 12 cases (20.3%), it was slight erythema and dry desquamation (G1); in 17 (28.8%), patchy moist desquamation and concomitant erythema (G2); in 25 (42.4%), confluent moist desquamation (G3); and in 5 (8.5%) patients, slight bleeding (G4) occurred.
Late toxicity was assessed during the entire observation period. The values given below relate to the occurrence of the highest observed toxicity levels during this time, and not to the state at the last follow-up visit. In one case (1.7%), there was no form of late toxicity; in 20 (33.9%), only depigmentation or hyperpigmentation of the treated area (G1) occurred; in 30 (50.8%), minor telangiectasias were found (G2), while massive telangiectasias (G3) occurred after treatment of 1 lesion (1.7%). Minor necrotic lesions (G4) were noted after treatment of 7 lesions (11.9%).
In the ReG, early toxicity was observed in all cases. In 7 (16.3%), it was slight erythema with dry desquamation (G1); in 18 (41.9%), patchy moist desquamation and concomitant erythema (G2); in 16 (37.2%), confluent epidermal desquamation was found (G3); and in 2 (4.6%), slight bleeding (G4) was noted.
In 1 case (2.4%), there was no late toxicity; G1 reaction as pigmentation disorders was seen after treatment of 20 tumors (30.2%); in 27 cases (62.8%), minor telangiectasias were found (G2); and massive telangiectasia (G3) occurred after treatment of 2 lesions (4.6%). There was no skin radiation-induced necrosis in this group.
No statistically significant differences were found in the frequency of degrees of early toxicity between the groups (p = 0.54). Additionally, the differences between the frequency of late complications degrees did not reach statistical significance (p = 0.16) despite the apparent difference in the occurrence of G4 toxicity between the groups.
Recurrence predisposing factors and increased toxicity analysis
Cox proportional hazards model was applied to evaluate the factors predisposing to recurrence. The characteristics of age, sex, overall treatment time (OTT), T feature, and specific locations were analyzed. Even though all recurrences in both the groups occurred in men and half concerned auricle tumors, due to too few complete observations, the Cox regression analysis failed. Therefore, based on the conducted study, it was impossible to determine the clinical factors predisposing to BCC recurrence after HDR-BT.
The factors predisposing to increased toxicity were also analyzed. Pearson’s χ2 test was used to analyze the influence of sex and the T feature, obtaining the level of statistical significance only for the T feature in the case of late reactions. However, since this variable did not meet the Cochran’s criterion, it was necessary to apply an additional statistical tool. Fisher-Freeman-Halton test confirmed the existence of a relationship of higher late toxicity in the treatment of T2 tumors (p = 0.028) compared with T1. Kruskal-Wallis test was used to investigate the effects of age and OTT. According to the analysis, these factors did not affect the occurrence of higher degrees of early reactions. For the late reactions, the study showed statistical significance only for OTT (p = 0.0499). However, in post-hoc analysis, this influence turned out to be not statistically significant. Due to many detailed locations and the uneven distribution of tumors within them as well as the reactions occurring, it was impossible to perform statistical calculations regarding the influence of this variable on the toxicity of treatment performed.
Discussion
Radiotherapy had played a significant role in skin cancers treatment for years, right from the moment of its’ invention [20, 21]. However, advances in surgical techniques, especially MMS, had led to a shift to the predominance of surgery as the most common method for skin cancer treatment. In the only prospective randomized clinical trial comparing both methods, Avril et al. demonstrated that the cosmetic effect was better, and the predicted 4-year local recurrence rate was lower with a standard POMA excision (0.7%) than with radiotherapy (7.5%). The mean follow-up was 41 months [22]. However, certain limitations cannot be overlooked when interpreting these results. The main eligibility criterion for surgical treatment was the presence of a previously untreated, biopsy-confirmed BCC in a facial area smaller than 4 cm. 173 patients were treated with tumor resection (94% T1, 6% T2, according to the current TNM system) with a healthy tissue margin of at least 2 mm. At the explicit request of the operator, intra-operative histopathological examination was performed in 91% of cases, resulting in decisions to increase the extent of tumor excision to obtain free margins in 67 patients. In 7 patients (5%), margins were still positive, but only six re-operations were done. This treatment seems to be a hybrid of POMA and MMS with a possibility of re-operation. Nonetheless, this complex procedure provided excellent LC comparable with the outcomes of MMS treatment [9].
One hundred seventy-two patients (92% T1; 8% T2) were treated with one of the three techniques depending on tumor and patient characteristics: low-dose-rate (LDR) interstitial BT with 192Ir (technique no longer used; 95 patients; dose range, 56-76 Gy in 6.9 days; BED, 65.4-90.9 Gy); contact therapy with 50 kV X-rays (technique no longer used; 57 patients; dose range, 34-40 Gy; mean BED, 100.8 Gy); or conventional EBRT with the use of 85-250 kV energy X-rays (20 patients; dose range, 33-65 Gy; BED, 78 Gy). Detailed information on therapeutic dose specifications was not provided. Therefore, it needs to be emphasized that only 20 patients were treated with the method still used today, which means that this prospective randomized trial did not stand the test of time and it is hard to compare its’ results to the current research. The study discrepancies affected the number of LRs. The highest recurrences reached 8.8%, and were recorded in the BT-LDR group with the most extensive BED spread. The LRs presented in our work were twice as low as in the study above (3.39%), with an average of two-months longer follow-up and a higher percentage of T2 tumors.
In a study on HDR-BT of primary BCCs, researchers from Valencia presented the results of 45 tumors in 32 patients [23]. Tumors were located on the scalp, trunk, and limbs in 82%, 10%, and 8% of patients, respectively, and the median maximum diameter of the lesion was 10 mm (range, 3-25 mm). All lesions were treated with Valencia-type contact applicators, with sizes chosen for the reference isodose 100% covering a 5 mm margin around the tumor. Tumor margins were determined based on a clinical examination or dermatoscopy. After assessing its’ thickness using ultrasound, the dose specification was made to a depth of 3 mm for lesions up to 3 mm thick, and a depth of 4 mm for lesions with a thickness between 3 and 4 mm. The treatment used a total dose of 42 Gy in two fractions a week (6 × 7 Gy in 96% of cases). The calculated BED was 71.4 Gy, and no LRs were observed during the median follow-up of 49 months. Only one treatment failure was reported for a lesion located on the scalp, due to an underestimation of the lesion depth. Despite the use of lower BED, longer OTT, and more sophisticated treatment planning (using ultrasound and dermatoscopy), the obtained LC was comparable to the present study. However, since most patients from the Tormo et al. group were treated for superficial T1 tumors, and in 18% of cases, the tumors were located outside the H&N area, there is a high probability that a significant part of the treated lesions could qualify as BCCs from the low-RR group [23].
Lower level of LR was obtained in one of the largest retrospective studies on BCC that analyzed data from the treatment of 862 primary BCCs, of which 841 (97.5%) were in the H&N region [24]. The treatment was carried out using two orthovoltage EBRT techniques (energy range, 29-100 kV), with fractionation 5 × 6.8 Gy every 2-3 days (OTT, 9-12 days; BED, 57.1 Gy). The authors did not provide precise data on the size of the treated tumors. A small proportion of them was characterized by a deep infiltration up to 17 mm, which can be deduced from the planning description that included dose-depth specification and tumor coverage with a peripheral margin of at least 5 mm. Based on the treatment results (estimated 5-year RR), we can conclude that in 43.6% of cases, the largest dimension of the treated lesions was < 10 mm. In this group, the ratio was 4.4%, and was significantly lower than 9.5% (p = 0.01) estimated in larger tumors. Other features did not influence the treatment results. For the entire study group, the 5-year RR was 7.4%, which is twice as high as in the current study (3.6%) for all tumor sizes, and more than two and a half times higher for tumors with largest dimension > 10 mm. Such result might be related to the use of significantly lower BED. The authors also compared the results of primary and recurrent BBC groups. Unfortunately, apart from the quantity (n = 211), no information was provided on the recurrent group characteristics The primary treatment method was not specified (it can only be concluded that it was not radiotherapy). The estimated 5-year RR was 9.5%, and the comparison did not show a statistically significant difference between treatment groups (p = 0.552).
The same authors published a retrospective study comparing SSE treatment effectiveness of primary BCCs (n = 588) and relapses after previous treatment (n = 135) [25]. The results were significantly worse in the group of secondary lesions treatment (4.8% vs. 11.6%; p = 0.034). The authors suggested that higher number of recurrences may be caused by non-radical resection as it may be challenging to define clinical tumor in tissues changed by the primary treatment. Operators’ attempt to minimize cosmetic defect could be another reason for non-radical resection. On the other hand, in case of any doubts, radiation oncologists may increase the irradiation field or specify the dose to a greater depth, thus ensuring the desired eradication of multipotent tumor cells and their remission. These results are consistent with the findings of Szewczyk et al., who compared the efficacy of classical SSE in the H&N region for primary (n = 204) and recurrent (n = 108) lesions. The percentage of recurrences in the group treated for recurrent BCC was more than twice as high as in the primary BCC group (20% vs. 9%), revealing that the infiltration of BCC recurrences may intensify, especially in the H&N region [26].
However, the location of tumor in the H&N region may also affect the results of ionizing radiation treatment, as shown by Wilder et al. in their study with 61 BCC recurrences after previous surgery, curettage-electrodesiccation, cryotherapy, radiotherapy, or a combination of these methods [27]. In the presented group, the tumors were diverse, and 70.5% of them occurred in the H&N region. They were treated with orthovoltage (range, 100-300 kV), megavoltage (> 1 MeV), or electron EBRT. Various fractionation schemes were applied, which did not facilitate its’ interpretation. With a median follow-up of 57 months, researchers obtained estimated 5-year complete remission rates of 96% for tumors with 5-10 mm dimension, and only 81% for larger tumors. On the other hand, for tumors located in the H&N region, this indicator was 88%, which was worse than 93% for other locations.
A report by Guix et al. should be mentioned, because it is the only one, in which the treatment concerns NMSC of the facial skin, and includes an analysis of recurrent cancers [28]. The presented group included 136 patients treated with the same number of tumors confirmed as BCC (n = 102) and SCC (n = 34). In the BCC group, 55 tumors were treated as primary lesions and 47 as relapses after previous surgery, while in the SCC group, it was 18 and 16 tumors, respectively. The authors did not provide information on the distribution of tumor sizes for either group. Leipzig applicators were used for 19 tumors smaller than 2 cm and located on flat surfaces, while in the remaining 117 cases, individual mold applicators were applied. The 100% reference isodose was located 5 mm from the applicator, and its’ reconstruction and planning were carried out using 2D X-ray imaging. In the case of tumors smaller than 4 cm, the treatment consisted of 33-36 daily fractions, 1.8 Gy each (total dose, 59.4-64.8 Gy; BED, 70.1-76.5 Gy), while lesions measuring 4 cm and more received a dose increased to 75-80 Gy after a 3-week break. The results of such treatment were excellent, but they were presented for all tumors together (BCC + SCC). With a 12-60 month follow-up, the estimated 5-year LC was 99% for patients with primary tumors, and 87% for patients with recurrent lesions. According to the commentary on the results, in the primary treatment group, only one SCC tumor relapsed, indicating that the effectiveness for primary BCCs was 100%. In the recurrent group, only 2 BCC tumors recurred. Statistical calculations focused solely on the group of patients with recurrent BCCs, and could reduce the presented results of local control in the form of Kaplan-Meier curves. The occurrence of only two failures out of 47 cases allowed us to obtain a crude recurrence rate of 4.25%, which is a slightly better result (by 0.4%) than the current study results in the ReG.
The fractionation applied in our study, resulting in relatively high BED for BCC cells, is most likely the reason for such a rare occurrence of treatment failure in both presented groups of patients. It allowed saving most patients from re-treatment, which is often associated with function deterioration and operated area cosmesis, or the subsequent, tedious reconstruction process. This is especially important when MMS surgery is not covered, while the available SSE with POMA are associated with a higher frequency of non-radicality, and thus often requires adjuvant treatment with radiotherapy [5, 29-31]. What is equally important, the treatment performed with HDR-BT does not exclude the possibility of salvage surgical treatment in the event of failure.
Despite its’ high effectiveness, the toxicity of 2D HDR-BT cannot be ignored. The treatment was discontinued due to acute toxicity just in one case. Such an intense reaction could be caused by an increased individual sensitivity to radiation, as described in the literature and observed with the use of regimens of much lower intensity [32, 33]. In the present study, the entire area irradiated, including the tumor site, was assessed one month after the treatment. The desired effect of treatment was tumor disintegration, resulting in a slight bleeding (grade G4) and ulceration, followed by gradual healing of the skin and epidermis. Therefore, it can be concluded that the presented method of early toxicity assessment represents rather a measure of regenerative abilities of the skin over four weeks after final irradiation. BED at the level of 75 Gy caused a toxicity > G2 persistence in 50.9% of cases from the PrG, and 41.8% from ReG.
Comparable results of early reactions one month after the application of BED in the range of 58.5-74.1 Gy were presented by Arenas et al. The study investigated 134 NMSC of the whole body (a very heterogeneous group containing primary lesions, recurrent lesions, and adjuvant treatment scars) treated with individual mold applicators (n = 33) and Leipzig applicators (n = 101). The G3 and G4 stages occurred in 42.5% of patients in total, and in 57.6% of patients treated with mold applicators (for tumors > 2 cm) [34].
Tormo et al. did not report early toxicity > G1, despite BED being lower by only 5 Gy compared with our scheme, but they did not provide information on when the reactions were assessed [23]. Guix et al. did not report > G2 reactions [28] using the LENT-SOMA classification [35], which is different from the RTOG/EORTC. They pointed out that 10% of patients had ulcers immediately after the end of treatment. The reaction was dose-independent, but related to tumor size, which is consistent with the observations of Arenas et al. Possibly, in the case of a larger group of patients in our study, the influence of tumor size on the severity of early toxicity could prove to be a statistically significant factor, as it turned out to be the only factor significantly influencing the increase in late radiation reactions.
In this study, the BED calculated for late reactions with the value of 133.3 Gy did not cause significant differences between both the groups in the incidence of complication rates (p = 0.16). It is important to mention that in the PrG, there were as many as seven cases of minor necrotic ulcerations (11.9%), while in the ReG, no G4 complication was observed. Perhaps, with a greater number of patients in the groups, a statistically significant difference would be revealed. That could be a good starting point for further research on potentially greater irradiation tolerance of the skin that underwent the healing process after surgical treatment.
Linear-quadratic model was used to compare different fractionation methods for both types of toxicity did not consider the total duration of radiotherapy treatment, nor the volume of irradiated tissues. Both the factors are extremely important in the context of skin tolerance to radiation therapy. This was demonstrated before the development of linear-quadratic model in experimental studies regarding the effect of field size, OTT, and dose fraction on the results and toxicity of skin cancer treated with EBRT [36, 37]. Based on these results, the calculation formula for the skin tolerance dose was established, in which all the three factors are crucial [38]. Therefore, if we assume that the tumor area (determined by the T feature) is directly proportional to the actual volume of irradiated tissues (the tumor and the surrounding healthy tissues), the statistically significant increase in late toxicity is dependent on the size of the lesions in our study, and confirms the effect of the field size on the skin tolerance also in the case of HDR-BT.
Our results (in both study groups) of late complications > G2 at the level of 9.8% indicate greater severity of the method compared with the mentioned reports on HDR-BT. However, it should be emphasized that none of these studies focused solely on treating BCC in the H&N region. Tormo et al. did not report any late reactions > G1, despite a higher calculated BED for late reactions (140 Gy), higher fractional dose, and a longer follow-up [23]. Guix et al. did not observe > G2 toxicity during the 5-year observation period. However, using 1.8 Gy fractionation reduced BED to the range of 95-125 Gy [28]. Arenas et al. observed > G2 toxicity in 3% of patients after applying a fractional dose of 3 Gy and BED in the range of 90-114 Gy, with a 33-month follow-up period [34]. The factor connecting these results is the longer OTT compared with our scheme. In our opinion, this again confirms the correctness of Prasad’s concept, indicating that prolonged treatment time may increase the dose tolerated by the skin [38]. Extended OTT can be achieved by lowering the fractionated dose with daily fractionation, as presented by Guix et al., or by hypofractionation at several days intervals, as proposed by Tormo et al. Extreme hypofractionation in the treatment of skin cancer was presented in a survey by Skowronek et al. Tumors of mixed histology were treated with a fractional dose of 10 Gy administered six times at intervals of 3-7 days. The BED for late reactions was as much as 260 Gy, yet no G4 late reactions were observed one year after the treatment [39].


