Introduction
Patients with locoregionally advanced melanoma constitute a specific group in regard to a high risk of recurrence and mortality. The involvement of lymph nodes and the presence of satellites characteristic for high-risk melanoma and in-transit metastases among stage III patients are associated with poorer prognosis and, despite the introduction of novel therapies, they remain a challenge [1]. Noticeable improvement of overall survival (OS) over the last years in a group of patients with advanced melanoma is related to several factors, including earlier diagnosis, improved surveillance with more effective detection of recurrences, the wide use of a sentinel node biopsy as well as novel therapies in palliative and adjuvant settings [2, 3]. Results from surgical studies, Multicenter Selective Lymphadenectomy Trial II (MSLT-II) and German Dermatologic Cooperative Oncology Group (DeCOG) proved that a surgical dogmatic approach that all sentinel node melanoma metastasis warrants completion lymphadenectomy is no longer valid [4].
Until the revolution and the introduction of novel regimens based on immuno- and molecular targeted therapy for over 20 years interferon α-2b was the only adjuvant regimen in resected high-risk melanoma [5]. Interferon treatment had a significant but unsatisfactory impact on the clinical outcome of high-risk melanoma and the improvement of OS and relapse-free survival (RFS) was approximately 3% [6]. Since 2011 a pool of new agents including the anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody (ipilimumab), programmed death-1 (PD-1) inhibitors (nivolumab, pembrolizumab), and BRAF/MEK inhibitors (vemurafenib, cobimetinib, dabrafenib, trametinib, encorafenib, binimetinib) have been registered by the European Medicines Agency (EMA) initially for the treatment of patients with unresectable or metastatic melanoma [7]. Despite the challenges in the treatment of advanced melanoma, the improvement of the response rates, progression-free survival (PFS) and OS has been achieved in recent years and the encouraging results prompted the development towards the use of novel agents in adjuvant settings in stage III melanoma patients after the radical resection [8–10]. Nivolumab and pembrolizumab were approved by the EMA as adjuvant therapies in the entire group of patients with resected stage III melanoma in July and December 2018, respectively [11]. Moreover, in August 2018, a combination of dabrafenib and trametinib was introduced in a group of completely resected stage III melanoma with the confirmed BRAF mutation [12]. Therapies mentioned above significantly improved patients’ clinical outcome and were also included in current European guidelines and nowadays are used in routine practice [13–15]. The reduction in the risk of the disease recurrence after administration of novel adjuvant therapies compared to placebo or standard treatment in patients with resected advanced melanoma, shown in recent clinical trials, ranges from 25% to 51% although their effect on OS is less evident [16–18].
However, a substantial part of the introduction of the novel adjuvant therapies was developed before the announcement of a new melanoma staging system. In 2018, the staging system was introduced and the stage III subgroups were re-separated from IIIA-C to IIIA-D [19]. From the point of view of the data presented in this article, the most important were changes in qualification criteria for stage IIIC and the addition of IIID subgroup as they are related to the highest risk of relapse and death due to melanoma and patients at these stages can benefit mostly from adjuvant treatment. Currently, qualification for stage IIID depends on the presence of ulcerated primary tumours larger than 4 millimetres (T4b) and simultaneous locoregional extensive tumour burden (N3a, N3b, N3c). Taking into account the aforementioned facts it seems to be particularly important to widely analyse current treatment results in these two emergent groups of patients, when the effective systemic therapy became available to patients with recurrent melanoma.
Aim
The main aim of this study was to analyse the contemporary clinical outcomes of the patients’ population with resectable stage IIIC and IIID melanomas (in accordance with the 2018 melanoma staging system) who were treated surgically before the introduction of the novel adjuvant therapies, but after introduction of the effective systemic therapy in metastatic relapse.
Material and methods
The study group
Out of the 283 initially enrolled patients, 10 patients were excluded from the statistical analysis due to significant shortcomings in the available clinic-pathologic data or the details of the treatment. The demographic, clinical, and pathological data of 273 consecutive patients treated between 2015 and 2018 for IIIC and IIID stages of melanoma were obtained from four Polish tertiary cancer centres (Wroclaw, Warszawa, Cracow, Gliwice). All patients enrolled in this study had histologically confirmed, resectable regional nodal metastases without in-transit or distant metastases at diagnosis. The population was divided into stage IIIC and IIID groups according to the eighth edition of the American Joint Committee on Cancer (AJCC) melanoma staging system [19].
Patients’ characteristics
The demographic and clinical characteristics of the study group were presented in Table 1. There were 127 females (F) and 97 males (M) in stage IIIC group (n = 224) and 49 patients in stage IIID melanoma (F : M, 16 : 39). In 197 (87.9%) patients with diagnosed IIIC melanoma the primary tumour arose from skin, in 5 (2.2%) patients from mucosa and in 22 (9.8%) patients the primary site was not found and the latter were classified as melanoma of unknown primary (MUP). Among the IIID melanomas, all but 2 (4.1%) lesions were of skin origin. Among the IIIC melanoma in 12 (5.4%) patients the primary tumour was located within the head and neck, in 93 (41.5%) patients within the trunk, in 29 (12.9%) patients within the upper limb, in 58 (25.9%) within the lower limb, and in 10 (4.5%) within other anatomic sites. In the IIID subgroup, the anatomic sites of the primary lesion were: in 4 (8.2%) cases head and neck, in 20 (40.8%) trunk, in 7 (14.3%) upper limbs, in 17 (34.7%) lower limbs and in 1 (2.0%) case different location. Within the IIIC stage group, 6 (2.7%) patients had recognized the T1 lesion, 24 (10.7%) patients had T2 lesions, 61 (27.2%) patients had T3 and 111 (49.6%) patients had T4 lesions. All of the patients (n = 49) with IIID stage melanoma had T4 lesions. Ulceration was present in 124 (55.4%) and 49 (100.0%) patients with IIIC and IIID melanomas, respectively. The presence of BRAF mutation was evaluated only in 172 (63.0%) patients. In Poland before 2018 BRAF mutation assessment was not obligatory in stage III melanomas due to the limited access to the novel systemic adjuvant therapies. Among the patients with known status of BRAF mutation, positive results were observed in 69 (51.1%) patients with IIIC and in 22 (59.5%) patients with IIID melanomas. Satellitosis was observed in 11 (4.9%) patients with IIID and in 11 (22.4%) patients with IIIC melanomas. In majority (86.0% in IIIC group and 60.0% in IIID group) the extracapsular invasion in the sentinel node was not observed. The lymphadenectomy after the tumour-positive sentinel node has been performed in 89 (39.7%) and 9 (18.4%) patients from stage IIIC and IIID groups, respectively. The other patients underwent the surgery due to clinically involved (palpable) lymph nodes (135 patients with IIIC and 40 patients with IIID melanomas). In stage IIIC patients the region of nodal involvement was: neck in 20 (8.9%), axilla in 117 (52.2%), groin in 84 (35.4%); in 3 (1.3%) two nodal regions were involved. Among the stage IIID melanomas, 4 (8.2%) patients underwent cervical, 24 (49.0%) axillary, and 21 (42.9%) inguinal lymphadenectomy. Bilateral lymphadenectomy was performed in 9 (4.0%) patients from stage IIIC group and in 2 (4.1%) from IIID stage group. Of IIIC patients, 60 (26.8%) had no further involved lymph nodes in the pathomorphological report, 67 (29.9%) had one, 59 (26.3%) had two or three and 38 (17.0%) had more than three. Among the patients with IIID stage there were 5 (10.2%) patients with two or three involved nodes and 44 (89.8%) with metastases in at least four lymph nodes. In factor analysis, patients were grouped into stages N1, N2, N3. There were 102 (45.5%) patients with N1, 75 (33.5%) patients with N2 and 47 (21.0%) patients with N3 in IIIC subgroup. In IIID subgroup all patients had stage N3.
Table 1
Demographic and clinical characteristics of the study group
Seventy-one patients, of whom 53 had stage IIIC (23.7%) and 18 (36.7%) had stage IIID melanoma, underwent postoperative radiotherapy. In the IIIC group, out of 83 patients who were treated in the palliative setting, 47 (56.6%) patients received immunotherapy, 21 (25.3%) patients received the targeted therapy, and in 12 (14.0%) other treatment, mostly based on dacarbazine, was applied. In 4 patients two lines of systemic therapy were administered. Among the 12 patients with IIID melanoma in whom palliative treatment was administered, 12 (66.7%), 5 (27.8%), and 1 (5.6.%) patient underwent immunotherapy, targeted therapy, and other treatment, respectively. The median follow-up time was 26.6 months (range: 1.7–67.2).
Ethical approval for research
The study was conducted according to the guidelines of the Declaration of Helsinki. As this was not an interventional or genetic study, ethical approval was provided by the Bioethical Committee at Maria Sklodowska-Curie National Research Institute of Oncology to release these data without additional patient consent as patient consent was deemed unnecessary (protocol code 3/2012 and date of approval: 18 December 2012).
Statistical analysis
The statistical analysis was performed using Statistica 13.3 software (TIBCO Software Inc, California, United States). The Kaplan-Meier method was used to assess the survival rates and the statistical significance was estimated based on the log-rank test. OS and RFS were calculated from the time of the diagnosis of lymph node metastases to the date of death and disease recurrence, respectively. Sex, age, primary tumour characteristics (location, tumour thickness, ulceration and BRAF mutation status), SLNB and lymphadenectomy details as well as radiotherapy were investigated in the univariate analysis. The variables with a p-value below 0.05 in univariate analysis were assessed as significant and they were included in multivariate analysis in which the Cox proportional hazard model was used.
Results
Overall survival
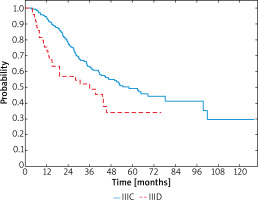
In the whole group, the median survival since the diagnosis of melanoma was 34.2 months (95% CI: 30.0–38.2) and the median relapse-free survival (RFS) was 17.4 months (95% CI: 14.9–21.2). At the time of the analysis, 130 patients, 102 (45.5%) in stage IIIC and 28 (57.1%) in stage IIID, were dead. Among IIIC stage patients median OS was 36.2 months (95% CI: 30.9–40.1) and in IIID stage patients it was 27.8 months (95% CI: 15.1–36.0). Hazard ratio (HR) for death was significantly lower among the IIIC patients (HR = – 0.57; 95% CI: 0.38–0.87; p = 0.014). The 1-year, 2-year and 3-year OS rates comparing stages IIIC versus IIID were 94% vs. 73%, 78% vs. 57% and 63% vs. 49%, respectively (Figure 1).
The median survival in patients in whom the recurrence was observed during the analysed period was 34.2 months (95% CI: 27.3–42.0) in those who underwent systemic therapy, and 25.6 months (95% CI: 20.1–31.5) in those who did not. In patients who received systemic therapy 3-year OS was 55% and for patients without systemic therapy it was 40% (Figure 2). In the group of patients receiving systemic therapy a significant reduction of HR was observed when compared to the untreated group (HR = 0.84; 95% CI: 0.58-1.22; p < 0.001).
Figure 2
OS curves in a whole study group stratified by receiving systemic therapy or not (p = 0.348)
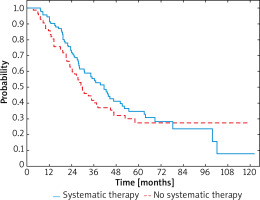
After dividing the patients depending on the stage of advancement similar correlations were observed in both groups. Comparing patients with recurrent IIIC melanomas, the median survival was 34.6 months (95% CI: 27.4–44.4) and 27.0 months (95% CI: 21.2–34.6) in those who were treated with systemic therapies and in those who were not, respectively. In patients who received systemic therapy 3-year OS rate was 55% compared to the 42% observed in patients who did not (Figure 3). Reduction of HR was also demonstrated between IIIC melanoma patients receiving systemic therapy or not (HR = 0.90; 95% CI: 0.60–1.36; p < 0.001).
Figure 3
OS curves in patients with IIIC melanoma stratified by receiving systemic therapy or not (p = 0.624)
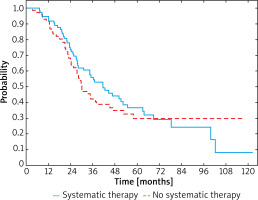
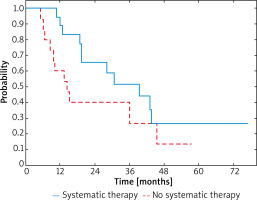
After recurrence of IIID melanoma, improved 3-year OS rate was also observed in patients after systemic treatment when compared to these untreated and was 52% and 27%, respectively (Figure 4). Median survival was 28.3 months (95% CI: 18.7–46.6) in the group treated with systemic therapy due to the relapse and 14.4 months (95% CI: 8.6–45.5) in the group with relapse and no systemic treatment. In patients who underwent systemic therapy HR was 0.54 (95% CI: 0.23–1.25; p < 0.001).
Relapse-free survival
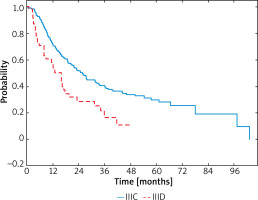
The disease recurrence was observed in 137 (61.2%) patients with the IIIC and in 33 (67.3%) patients with IIID melanomas. Locoregional recurrence was reported in 40 (29.2%) and 11 (33.3%) patients with IIIC and IIID melanomas, respectively. Ninety-seven (70.8%) patients from the IIIC subgroup and 22 (66.7%) from the IIID subgroup had distant metastases. Patients with stage IIIC had improved outcome (HR = –0.51; 95% CI: 0.35-0.75; p < 0.001), with a median RFS of 19.7 months (95% CI: 16.2–23.7) compared with stage IIID in which the median was 8.9 months (95% CI: 4.7–16.3). In patients with stage IIIC melanomas 1-year RFS rates were 72%, 2-year RFS – 51% and 3-year RFS – 39% and, in contrast, in IIID melanomas these rates were 58%, 29%, 13%, respectively (Figure 5).
Uni- and multivariate analysis for OS
Univariate analysis of negative prognostic factors for OS in patients with stage IIIC and IIID melanomas was performed with seventeen variables (Table 2). A significant determinants of poorer OS were sex (p ≤ 0.004), age (p = 0.022), tumour thickness (p = 0.001), ulceration (p = 0.039), satellitosis (p = 0.004), number of involved nodes at the pathology report on lymphadenectomy specimen (p = 0.038) as well as lymphadenectomy location (p = 0.014) and N stage (p = 0.006). In multivariate analysis using a Cox proportional hazards model and stepwise regression, the negative predictors which met the criteria of the statistical significance were male sex (HR = 1.89; 95% CI: 1.30–2.74; p = 0.004), age of 65 and over (HR = 1.53; 95% CI: 1.07–2.17; p = 0.022) and presence of satellitosis (HR = 2.23; 95% CI: 1.31–3.78; p = 0.023).
Table 2
Uni- and multivariate analysis for overall survival (OS) among IIIC and IIID melanoma patients
Uni- and multivariate analysis for RFS
As in the analysis of prognostic factors affecting OS, seventeen factors were taken into account in case of RFS. The univariate analysis identified sex (0.001), ulceration (0.031), BRAF mutation (< 0.001), satellitosis (< 0.001), sentinel node biopsy (0.029), lymphadenectomy type (0.036), number of involved nodes at the pathology report on lymphadenectomy specimen (< 0.001) and N stage (< 0.001) as significant prognostic factors. In a multivariate Cox regression model, negative prognostic factors for RFS were male sex (HR = 1.41; 95% CI: 1.03–1.96; p = 0.033), presence of BRAF mutation (HR = 2.78; 95% CI: 1.85-4.17; p < 0.001), presence of satellitosis (HR = 2.38; 95% CI: 1.47–3.85; p < 0.001) and N stage (N2 – HR = 1.56; 95% CI: 1.06–2.27 and N3 – HR = 2.04; 95% CI: 1.43–2.94; p = 0.021) (Table 3).
Table 3
Uni- and multivariate analysis for relapse-free survival (RFS) among IIIC and IIID melanoma patients
Discussion
Recent studies have shown convincing evidence for improvement of the clinical outcome within the patients with advanced melanomas undergoing a novel adjuvant therapy [20]. The PD-1 inhibitors and BRAF/MEK inhibitors intended be used in an adjuvant setting significantly lower the risk of recurrence, death and the probability of development of distant metastasis [21, 22]. The encouraging results obtained in the initial trials allowed the introduction of these methods to clinical practice, however, they should be reviewed and carefully analysed in view of the new staging system which mainly concerned stage III melanomas.
High-risk melanomas are associated with equal to or greater than 50% risk of recurrence or death in 5-year perspective [23]. The introduction of the 8th AJCC melanoma staging system caused controversies, nonetheless, it allowed to divide more accurately subgroups at high risk of relapse and death from the heterogeneous stage III group [24, 25]. The predicted 5-year OS in accordance with the 7th AJCC staging system varied from 78% to 40% (IIIA-C) and after the 8th update from 93% to 32% (IIIA-D) [19]. In the direct comparison between IIIC and IIID stages, 5-year OS was significantly higher in the IIIC melanomas (69%) than in IIID (32%) whereas IIID stage was rather comparable to stage IV [26, 27]. The increasing stage of the disease advancement, in accordance with novel staging, particularly affects the RFS as well and among stage IIIC 1-year RFS about 50% and among IIID about 30% was reported [28]. Especially high rates of recurrence and risk of death shortly after the lymph node dissection was observed in IIID melanoma, however, significant improvement of the clinical outcome, reported in recently completed clinical trials under novel therapies in adjuvant settings, seemed to indicate that adjuvant treatment is justified and can provide a decreased risk of the disease relapse in both IIIC and IIID groups [29]. It should be noted that patients who suffered from stage IIID melanoma constitute a minority within stage III (4–5%), however, due to the poor prognosis, the analysis of this group seems to be particularly important [30].
In this study, special attention was paid to the group of patients with locoregional advanced melanoma in whom there were both major changes in classification and treatment in recent years, but the adjuvant therapy has not yet been available. Among the presented study sample, OS rates observed in IIIC and IIID groups were slightly improved than in patients analysed by the AJCC Expert Panel [26]. The reason is twofold: first, in the presented study the patients had access to novel palliative therapies after the recurrence, and second, patients with in-transit metastasis were excluded from the analysis. Nonetheless, both the poorer prognosis in patients with stage IIID melanomas and the significant improvement in the clinical outcome in the group of patients who underwent novel palliative therapies at relapse were confirmed. Analysis of independent factors for worsening OS and RFS outcomes in patients with locoregionally advanced melanoma was widely described and our study confirmed that demographic factors such as sex and age are significant in this specific group [31–33]. However, only male gender, age over 65, and the presence of satellitosis were negative risk factors for OS confirmed by the multivariate analysis in this study. The particularly important prognostic factors affecting RFS, as confirmed by the multivariate analysis, are male sex, presence of BRAF mutation, presence of satellitosis and increase in N stage. The stratifying risk in patients with stage III melanomas is especially important and the novel diagnostic methods, on par with statistical data, may enhance this process and reduce the side effects of novel therapies in a group of patients who will not benefit from the treatment [34].
Conclusions
The survival of patients with locoregionally advanced melanomas is significantly better than that observed in the historical groups, however, it is still unsatisfactory. We have analysed the current outcomes of the newly distinguished highest risk subgroups of stage III melanomas: IIIC and IIID and confirmed clear differences between them in terms of prognosis, poor RFS but significantly better OS as compared to this observed in the original AJCC cohort, what may be related to the impact of introduction of novel active therapies used at the disease relapse. In a retrospective analysis of patients with recurrent melanoma, an improvement in life expectancy and a reduction in HR were observed in the group treated with systemic therapies. The results of this analysis show sex, age, tumour thickness, ulceration, satellitosis, different lymphadenectomy location, number of involved nodes reported in the lymphadenectomy specimen and N stage as important prognostic factors for OS in patients with IIIC and IIID melanoma. Similarly, sex, ulceration, BRAF mutation, satellitosis, sentinel node biopsy, lymphadenectomy type, number of involved nodes reported in the lymphadenectomy specimen and N stage were significant prognostic factors for RFS. Successful prevention and treatment of metastatic disease, mostly due to adjuvant therapies developed in recent years, should in the nearest future synergistically improve patients’ survival.