Purpose
Primary liver cancer (PLC) accounts for 8.2% of all cancer deaths worldwide, and is the fourth most lethal malignancy [1, 2]. There are 782,000 deaths and 841,000 new cases of PLC worldwide every year, with East Asia being the region with the highest prevalence of the disease [1]. Hepatocellular carcinoma (HCC) constitutes for 85-90% of primary liver malignancies, and is the fourth most prevalent and the second most deadly cancer in China [3].
Barcelona clinic liver cancer (BCLC) classification has been widely validated. Due to variability of HCC prevalence, phenotype, and treatment response in China, specific guidelines for Chinese patients have been established [4]. China liver cancer staging (CNLC) system was found in 2017, and has been used since then. In CNLC system, each stage of BCLC 0/A, B, and C is divided into two sub-stages, including stages Ia, Ib, IIa, IIb, IIIa, and IIIb [5].
Since there are many blood vessels in liver cancer tumors, and most of blood supply comes from the hepatic artery, intra-arterial therapy is the mainstay of treatment for intermediate stage liver cancer [6, 7]. Globally, trans-catheter arterial chemo-embolization (TACE) has been acknowledged as the gold standard for the treatment of intermediate stage liver cancer, with survival rates ranging from 20 to 36 months [8]. The procedure has been significantly improved with a principle of selectivity in the injection process, minimizing the risk of non-targeted embolization and liver failure. In addition, internal radiotherapy for HCC involves primarily iodine-125 (125I) seed implantation and trans-arterial radio-embolization [9]. Iodine-125 has a half-life of 59.6 days, which ensures that tumor cells are killed over a longer period of time. The radiation from 125I is mainly X-ray and γ-ray to induce DNA mutation and/or damage of tumor cells, and to generate tumor cells apoptosis [10]. Iodine-125 implantation is usually combined with other treatment modalities in the treatment of HCC [11, 12]. The combination of TACE and 125I implantation (TACE-125I) has been shown to be more effective than TACE alone in prolonging life of patients with different stages of HCC [13, 14].
For CNLC stage IIIa primary liver cancer, most patients have no indication for surgical resection [15], and for those with good liver function, the Chinese liver cancer staging system recommends TACE as the preferred treatment [5], in addition to systemic anti-tumor therapy (chemotherapy, targeted and immunotherapy) and radiotherapy (internal and external radiotherapy), which can be applied alone or in combination. The combination of TACE with targeted and immunotherapy treatment has significantly prolonged progression-free survival and overall survival [16-19]. For patients with type III portal vein tumor thrombus (PVTT), there are few reported cases who underwent radical treatment, and remained recurrence-free for a long time. In the present study, we report on a case, in which radical treatment was achieved by TACE combined with radioactive 125I particle implantation in portal vein tumor thrombus, with subsequent oral anlotinib hydrochloride capsules.
Case presentation
This study was approved by the Ethics Committee of Affiliated Hospital of Youjiang Medical College for Nationalities. Written informed consent was obtained from the participant.
A 55-year-old male patient was admitted to the hospital on January 1, 2020 with epigastric pain for more than 1 month. The patient had been drinking home-brewed yellow wine for more than 20 years (about 250 ml a day), and had no other associated diseases. Numeric rating scale (NRS) score was 5, and performance status (PS) score was 1. Additional tests (performed on January 3, 2020) showed ALT = 74 U/l, AST = 220 U/l, liver function Child-Pugh score = 7 (human blood albumin, 25 g/l, total bilirubin, 13.3 μmol/l, PT 12.5 s, no ascites or hepatic encephalopathy), AFP > 1,210 ng/l, and CA199- and CA125-negative. Computed tomography (CT; done on January 6, 2020) results showed multiple hypodense foci seen in the right lobe of liver, involving right branch of portal vein and a part of portal trunk (Figure 1). The patient was clinically diagnosed with 1) PLC (stage IIIa CNLC, PVTT Cheng’s type III), 2) Mild cirrhosis, and 3) Chronic viral hepatitis B.
Fig. 1
Computed tomography (CT), DSA images before treatment. A) Cross section at CT arterial stage. B) Cross section at CT portal stage. C) CT portal stage (multiple hypodense lesions with irregular morphology and no obvious envelope seen in the right lobe of the liver, measuring approximately 16 cm × 9 cm. CT diagnosis showed (1) Diffuse hepatocellular carcinoma in the right lobe of liver, with invasion of the right branch of portal vein and part of the trunk, and (2) Mild cirrhosis. D) Angiography of DSA before treatment. DSA hepatic arteriography showed multiple blood-rich lesions in the right lobe of liver, without arterio-venous fistula or arterio-venous portal veins. The white arrow indicate the hepatocellular carcinoma lesion, and the black arrow is the portal carcinoma thrombus
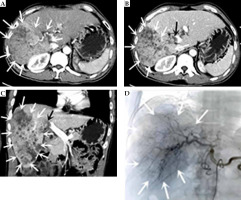
Drug-eluting bead TACE (D-TACE) combined with conventional TACE (C-TACE) was performed on January 9, 2020. DSA hepatic arteriography showed multiple blood-rich lesions in the right lobe of the liver, without arterio-venous fistula or arterio-venous portal veins. Tumor-supplying artery was super-selected by microcatheter; a chemotherapeutic solution was prepared by adding 50 mg of lobaplatin to 30 ml of 0.9% sodium chloride solution, and chemotherapy was administered by infusion through the microcatheter for 20 min. Pirarubicin 40 mg was then added to a vial of drug-loaded microspheres (diameter, 100-150 μm), loaded for 15 min. Next, 7 ml of iohexol was applied to form a suspension, which was slowly infused through the microcatheter. The tumor was then embolized with pirarubicin 10 mg and iodized oil 10 ml, followed by further embolization with gelatin sponges (diameter, 750-1,000 μm) until tumor’s supply artery was completely occluded. Anlotinib hydrochloride capsules 12 mg (QD) D1 to D14 were administered orally from the 3rd day after surgery and continued for 1 week.
At follow-up date, February 24, 2020, the patient’s spirit and appetite had improved, and NRS score was 3. Enhanced CT showed a reduced lesion in the right lobe of the liver, but poor control of the portal vein cancer thrombus (Figure 2B). The overall assessment was that the lesion was well-controlled. After a multidisciplinary consultation and discussion, the patient underwent a minimally invasive 125I particle implantation intervention on February 26, 2020, with six 125I particles (average energy, 35.5 KeV per particle; half-life, 56 days; radioactivity, chemical 0.7 mCi; tissue penetration capacity, 1.7 cm). Based on pre-operative CT scans, the approximate dose distribution of solid tumors at the prescribed dose was simulated using treatment planning system (TPS, Beijing Hanglin Technology Development Co., Ltd., China), and the number and spatial distribution of particles (V100 ≥ 95% and D90 ≥ 100%) were determined by designing a linear array of radioactive particles in the center of cancer thrombus cross-section, extending along the long axis of cancerous thrombus (long axis of portal vein). Total irradiation dose was 120 Gy. Under ultrasound positioning, an 18 G eight-light needle was used to percutaneously puncture the center of cross-section of the cancerous thrombus, along the long axis of the right branch of parallel portal vein to 4 mm from the end of main stem of the cancerous thrombus. Radioactive particles were implanted continuously and linearly, with no space between particles. After implantation, the eight-light needle was left in place, and a CT scan of upper abdomen was performed for any possible sub-peritoneal bleeding, large vessels, and a particle displacement or gas embolism in the heart. After the particle implantation reached the expected plan, the eight-light needle was removed, pressure was used to stop the bleeding, and bandages were applied. Instruments used for this procedure included a pistol-type particle implantation device, an 18 G particle implantation needle, and 125I radioactive particles with an activity of 2.22E + 7 Bq (Beijing Atomic Technology Co., Ltd., China).
Fig. 2
Computed tomography (CT)-enhanced cross-sectional images of the portal phase before and after treatment. A) Before treatment. B) After more than 1 month of treatment. C) After more than 8 months of treatment. D) After more than 15 months of treatment. E) After more than 23 months of treatment. The white arrow indicate the gradually shrinking liver cancer lesion, the black arrow is the portal cancer thrombus, and the red arrow is the radioactive 125I particle
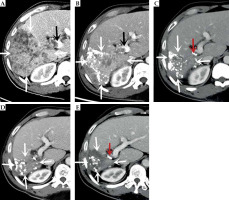
C-TACE was performed again on March 2, 2020, June 24, 2020, September 27, 2020, and December 17, 2020. The results of DSA hepatic arteriography showed that the right lobe of the liver was further reduced, and there was no arterio-venous fistula. The tumor-supplying artery was super-selected by microcatheter, and a chemotherapeutic solution was prepared by adding 50 mg of lobaplatin to 30 ml of 0.9% sodium chloride solution; chemotherapy was administering by infusion for 20 min via microcatheter. Pirarubicin 20 mg and iodized oil 10 ml was then used to prepare a suspension and embolized through the microcatheter, with additional gelatin sponge (diameter, 750-1,000 μm), until the tumor donor artery was completely embolized. The tumor was treated with oral anlotinib in a sequential manner. CT re-examination showed that the lesions in the liver gradually decreased, the portal vein gradually atrophied, a part of the portal vein opened, blood flow resumed, and the left lobe of the liver gradually increased. AFP level showed a continuous decrease (as of December 17, 2020).
On April 8, 2021 (after more than 15 months of treatment), the patient’s pain disappeared, NRS score was 3, his weight increased by 10 kg, and PS score was 0. CT results showed complete necrosis and no enhancement of the intra-hepatic lesion, atrophy of the portal vein, partial opening of the main trunk, and the right branch of the portal vein to restore blood flow (Figure 3). AFP level decreased to normal range (1.96 ng/ml). The patient returned to the hospital for follow-ups on July 28, 2021, December 26, 2021, July 25, 2022, and March 23, 2023, with no complaints of discomfort (PS score, 0). AFP values maintained within normal range, and CT results indicated no recurrence or metastasis. At the last follow-up on March 23, 2023, the patient had a pain NRS of 0, weight 68 kg, PS score 0, AFP 1.96 ng/ml, liver function Child-Pugh score 5 (human blood albumin 38 g/l, total bilirubin 6.2 μmol/l, PT 11.6 s, no ascites or hepatic encephalopathy). After extensive evaluation, the patient’s disease was completely controlled, with no long-term recurrence or metastases and radical outcome. To date, PFS is 37 months.
Fig. 3
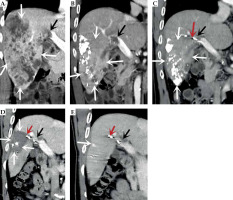
Computed tomography (CT)-enhanced coronal images of portal phase before and after treatment. A) Before treatment. B) After more than 1 month of treatment. C) After more than 8 months of treatment. D) After more than 15 months of treatment. E) After more than 23 months of treatment. The white arrow indicate the liver cancer lesion, which is gradually shrinking, the black arrow is the portal cancer thrombus, which is gradually shrinking and disappearing, and the portal vein is partially open, the red arrow is the radioactive 125I particle
Discussion
Primary liver cancer is one of the major diseases that seriously threaten human health. With a high-rate of prevalence, is the fourth most common malignant tumor in China. Furthermore, it has a poor prognosis, and is the second leading cause of neoplastic mortality [20]. Approximately 800,000 people worldwide die due liver cancer each year, with China accounting for nearly half of these deaths [21, 22].
Guangxi is a region with a high incidence of liver cancer in China. When detected, the majority of individuals with HCC are already in the mid-to-late stages due to insidious onset, high malignancy, and rapid progression of the disease; 40-60% of these patients have additional portal vein carcinoma thrombosis [1, 6, 23]. CNLC stage IIIa PVTT is highly aggressive, and is an important cause of tumor metastasis, liver failure, and fatal bleeding from ruptured esophago-gastric fundic varices. The median survival of patients with primary liver cancer is only 2.7-4 months [24].
For hepatocellular carcinoma combined with portal vein cancer thrombosis, TACE can infuse high concentration of chemotherapeutic drugs and embolize tumor blood vessels to effectively control the lesion, but the effect of controlling portal vein cancer thrombosis is limited, and targeted treatment of portal vein cancer thrombosis is needed to prevent further spread of portal vein cancer thrombosis, leading to an increased portal hypertension causing lethal complications, such as gastrointestinal bleeding [25, 26].
Patients with mixed portal cancer thrombosis, in which liver cancer cells are already present in the circulation, require systemic therapy. Anlotinib hydrochloride is a novel, potent, and multi-targeted small molecule tyrosine kinase inhibitor that can fully engage relevant targets in angiogenic pathway, including PDGFR, VEGFR, and FGFR. As an anti-angiogenic agent, anlotinib is effective as a single-agent therapy with few side effects [27] that is able to control tumor cell proliferation and metastasis by inhibiting c-Kit [28, 29], Ret [30], FGFR [31, 32], c-Met [33], and other targets. Anlotinib alleviates immunosuppression, increases T-cell activity and infiltration, coordinates immunotherapy [34, 35], increase local oxygen partial pressure and oxygen content in tumors, inhibits radiotherapy-induced angiogenesis and sensitizes radiotherapy, and achieves re-modeling of therapeutic micro-environment. Its combined potentiating effect makes TACE combined with portal vein radioactive particle implantation more effective. A number of studies [19, 36-38] have shown that anlotinib has a better anti-hepatocellular carcinoma effect with relatively few side effects, and more patients can tolerate it for a long time.
Guo et al. explored the efficacy and safety of TACE in combination with anlotinib (TC) compared with TACE alone (TA) in patients with unresectable hepatocellular carcinoma. PFS was found to be significantly higher in the TC group than in the TA group (7.35 months vs. 5.54 months) [36]. Wang et al. studied 145 consecutive patients with HCC treated with TACE and radiofrequency ablation (RFA). Twenty-eight of these patients were diagnosed with PVTT and received anlotinib as the primary treatment. The results showed a median overall survival (OS) of 13 months (3~18 months), and a 1-year OS rate of 64.3% [19]. A recent single-center, randomized controlled phase II study involved 38 HCC patients with BCLC stage B or C and ECOG PS ≤ 1. The enrolled patients were treated with TACE combination with anlotinib and TACE alone. Eighteen of these patients received TACE in combination with anlotinib, and twenty patients received TACE alone. The results showed a median PFS of 11.04 months in the combination group, and 6.87 months in the TACE group. Disease control rate (DCR) was 94.4% and 100%, respectively [39]. In the present study, we reported on a male patient with stage IIIa primary hepatocellular carcinoma and portal vein carcinoma thrombosis Cheng’s type III, who was treated with TACE combined with radioactive 125I particle implantation, followed by oral anlotinib hydrochloride capsules, and achieved a current progression-free survival of 37 months, which was superior to the efficacy of TACE combined with anlotinib. However, this was a single-center trial, and further studies with more patients are needed to investigate the efficacy of this method in the treatment of primary liver cancer.
TACE combined with portal 125I radioactive particle implantation and subsequent anlotinib-targeted combination therapy, can rapidly reduce tumor, open portal vein, reduce portal pressure, decrease fatal complications of gastrointestinal bleeding, alleviate symptoms of portal hypertensive gastropathy and enteropathy, improve appetite, and help improve immunity. It provides an option for a combination treatment strategy with significant efficacy for patients with primary liver cancer (CNLC stage IIIa, PVTT Cheng’s types II and III), but requires retrospective or prospective studies to further confirm clinical efficacy.
In the current study, we presented a case of a male primary hepatocellular carcinoma stage IIIa and PVTT Cheng’s type III, who was treated with TACE combined with portal carcinoma thrombosis radioactive 125I particle implantation and subsequent administration of oral anlotinib hydrochloride capsules. Fifteen months later, radical cure was achieved, and current progression-free survival is 37 months.


