Introduction
Cutaneous T-Cell Lymphomas (CTCLs) represent a group of extra nodal non-Hodgkin lymphomas, which arise from malignant clonal transformation of T-cells in the skin. While CTCL primarily affects the skin, it may subsequently involve blood, lymph nodes and visceral organs. The most common subtype of CTCL is mycosis fungoides (MF), which according to WHO-EORTC classification accounts for approximately 60% of cases [1]. The symptoms of MF include patches, infiltrating plaques which can progress to tumours as well as erythroderma. Lymph nodes, blood and visceral involvement may follow the skin lesions in the minority of cases [1]. Sezary syndrome (SS) is an aggressive form of CTCL, comprising approximately 5% of CTCL cases [2]. It is defined by a triad: erythroderma, lymphadenopathy and clonal neoplastic proliferation of T-cells in the blood [1, 2]. Treatment of early-stage CTCL includes skin-directed therapies such as topical steroids, topical cytostatic agents and phototherapy [3, 4]. The advanced stage of CTCL is treated with systemic therapies such as interferon α, bexarotene, chemotherapeutic agents, extracorporeal photopheresis, allogeneic hematopoietic stem cell transplantation (allo-HSCT) and others [3, 4]. CTCL is accompanied by persistent pruritus that affects between 60% and 90% of patients [5, 6]. It is difficult to treat as antihistamines are usually ineffective and thus pruritus significantly reduces quality of life. Therefore, it seems that a non-histaminergic pathway is involved in pathogenesis of CTCL’s pruritus.
Four-helix bundle cytokine, interleukin-31 (IL-31), is a non-histamine pruritogen that is proven to be correlated with pruritus in atopic dermatitis [7, 8]. It belongs to the IL-6 cytokine family that includes IL-6, IL-11, IL-27, oncostatin M (OSM), ciliary neurotrophic factor (CNTF), leukaemia inhibitory factor (LIF), cardiotrophin-1 (CT-1) and cardiotrophin-like cytokine (CLC), all of which share glycoprotein 130 (gp130) as a receptor subunit [9–11]. In contrast, IL-31 uses IL-31 receptor α (IL-31 RA). Hence, IL-31 signals through a heterodimeric receptor consisting of IL-31 RA and oncostatin M receptor β (OSMR) [9–11]. Through that receptor complex, IL-31 induces the activation of Janus kinase/signal transducer and activator of transcription (JAK/STAT), mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase/AKT (PI3K/AKT) signalling pathway, which results in translocation to nucleus and then gene transcription [10, 11]. IL-31 is produced by a variety of cells, predominantly by CLA+ CD45RO+ memory T cells and also mast cells, monocytes/macrophages, dendritic cells [9–12]. T helper 2 cells (Th2), a subtype of T-cells, were demonstrated to be the primary source of IL-31 in human skin diseases [12–14]. The predominant tissues expressing IL-31 mRNA are skin, lung, nervous system and intestine ones [9–11]. IL-31 mRNA was also found in the testis, bone marrow, skeletal muscle, kidney, thymus, colon, trachea and dorsal root ganglia (DRG) [9, 10, 13]. IL-31 RA and OSMR are co-expressed on keratinocytes, activated monocytes and DRG [9, 10, 13, 14]. However, constitutive expression of both receptor units is found in many other tissues such as thymus, testis, ovary, prostate, placenta, spleen, trachea, bone marrow ones suggesting that IL-31 may have potential pleiotropic physiological functions [9, 10].
According to recent research results, interleukin-31 (IL-31) is involved in the inflammatory process and initiation of pruritus in pruritic skin dermatoses indicating that a blockade of the IL-31 pathway may be an alternative to antihistamines in itch treatment. Moreover, recently, an anti-IL-31 receptor antibody has been shown to significantly improve pruritus in patients with atopic dermatitis. As CTCL and AD share some similarities, IL-31 has been suspected to play an analogous pruritogenic role in CTCL. However, the quantity of research on IL-31 role in CTCL is rather limited and conclusions are not homogeneous. CTCL shares many clinical, histological and immunological characteristics with AD. Both diseases show infiltration of the skin by skin homing T cells, predominance of Th2 immune response and pruritus [15–17]. Due to the fact that pruritogenic function of IL-31 in atopic dermatitis is well established, its role in CTCL remains a promising target of investigation. In our work we have described the IL-31 role in AD and focused on publications concerning the pruritogenic role of IL-31 in CTCL and its potential association with the stage of the disease.
Aim
As there are several excellent systematic reviews comprehensively dealing with IL-31 role in AD [18, 19], we conducted a systematic review of only CTCL.
Material and methods
An extensive search in the literature was performed by two investigators for studies that approached the association between IL-31, pruritus and CTCL in humans. We performed a comprehensive literature review in line with the criteria published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Science Direct and Web of Science were the medical and scientific databases used in the literature retrieval. We used the following combination of searched key words: “interleukin-31”, “cutaneous T-cell lymphomas”, “mycosis fungoides”, and “Sezary syndrome”. Inclusion criteria: studies included IL-31 correlation with pruritus and/or a disease stage of CTCL, case-control research, no duplicated data in the study. Articles concerning diseases other than CTCL, pertaining to signalling pathways, biochemical properties, treatment association with IL-31 levels, animal studies, reviews, case reports, and personal experience summaries, studies not meeting the inclusion criteria of this study or in a language other than English were excluded. Relevant articles were screened by title and abstract, then the full text was assessed for eligibility.
Results
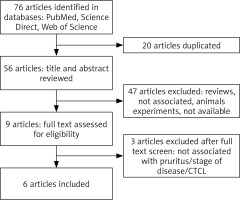
The flow diagram of this study is presented in Figure 1 [20]. We identified 76 potential studies and publications, 20 were excluded for duplication, 47 were excluded after title and abstract screening, while 3 were excluded after full text screening. Furthermore, articles were excluded for diseases other than CTCL, lack of relevance to pruritus or stage of the disease, review articles or animal studies. Key studies and findings are highlighted in Table 1.
Table 1
Summary of interleukin-31 studies in CTCL regarding the IL-31 serum level or expression in the skin
| Reference | Conclusions |
|---|---|
| Ohmatsu (2012) [53] |
|
| Nobbe (2012) [40] |
|
| Singer (2013) [52] |
|
| Mobs (2014) [54] |
|
| Malek (2015) [51] |
|
| Nattkemper (2016) [50] |
|
Solid pruritogenic role of IL-31 in atopic dermatitis
Recent studies have been continuously extending knowledge of the complex pathophysiology of pruritus involving complicated interactions not only between multiple skin cells and the afferent nerve fibres but also new pruritogens, such as IL-31 and non-histaminergic receptors (Figure 2) [21–25]. However, the mechanism of pruritus brought by those mediators is still not known. IL-31 is a pleiotropic inflammatory cytokine involved in regulation of the inflammation and inducing pruritus [9–11]. As IL-31 RA and OSMR, subunits of the IL-31 receptor, are expressed in dorsal root ganglia and epidermis, IL-31 is indicated to be a missing link between the neural and immune system in the induction of inflammatory itch. Series of research on transgenic mice showed that overexpression of IL-31 resulted in atopic-like dermatitis [13, 26]. Another evidence of the pruritogenic role of IL-31 is induction of pruritus after IL-31 cutaneous injection [13]. Dillon et al. demonstrated that cutaneous injection of IL-31 in mice elicited pruritus after a couple of days, moreover pruritus persisted for a few days after administration had stopped [13]. Both intradermal and intravenous administration of IL-31 showed delayed onset of itch and long-lasting scratching behaviour in mice [27, 28]. Similarly, IL-31 induces transient scratching behaviour in primates-cynomolgus monkeys [29]. When it comes to humans, Havro et al. study indicated that IL-31 does not induce an immediate itch after a skin challenge [30]. Interestingly IL-31 administration caused strong and long-lasting erythema in almost all AD patients and healthy subjects suggesting its role in promoting local inflammation [30].
Figure 2
Schematic diagram of the pruritus pathway with main producers and itch mediators. The signal following the binding of itch mediators (pruritogens) to specific receptors (pruriceptors) from the skin is transmitted by itch-selective C-fibres (histamine-dependent itch fibres and histamine-independent itch fibres) to dorsal root ganglia and spinal neurons in lamina I of the dorsal horn, the itch signal ascends in the contralateral spinothalamic tract which projects to the thalamus. Then itch is transmitted to the somatosensory cortex, involved in recognition of itch
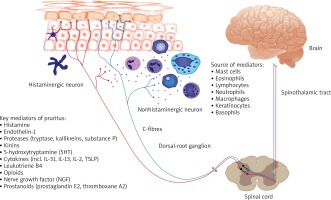
Lack of induction of immediate itch after IL-31 administration in AD patients or healthy controls suggests that IL-31 acts indirectly via keratinocytes, secondary mediators and probably then C-fibres activation rather than its own receptors on skin neuronal endings. A recent study by Cevikbas et al. demonstrated that some itch neurons co-expressed transient receptor potential cation channel vanilloid subtype-1 (TRPV-1), transient receptor potential cation channel ankyrin subtype-1 (TRPA-1) and IL-31RA as targets for IL-31 Th2 mediated itch [14]. Jianghui Meng et al. showed for the first time that IL-31 increased synthesis of brain-derived natriuretic peptide, an itch mediator, in skin and DRG in AD [31]. It indicates a link between IL-31 mediated pruritus and sensory neurons. Several excellent reviews have covered the mechanisms and involvement of the IL-31 and its receptor axis in pruritus and inflammation process [32–34].
Kasutani et al. showed that intravenously administered IL-31 receptor antibody reduced itch in mice [28]. In a separate study by Grimstad et al., anti-IL-31 antibodies reduced itch in a murine model of atopic dermatitis [26]. In primates as in mice, the IL-31-induced scratching was suppressed by anti-human IL-31RA monoclonal antibody [29]. These studies elicited clinical trials (phase 1 and phase 2 clinical) in AD on human monoclonal interleukin-31 antagonist, nemolizumab (CIM331), which turned out to decrease pruritus [35]. The results have shown pruritus reduction up to 50% (visual analogue scale, VAS) after 4 weeks’ use of antibodies [35]. Moreover, AD patients treated with CIM331 had improved sleep efficacy and required less topical hydrocortisone use [35]. A phase 2 trial comparing effects of nemolizumab with placebo found that the monthly administration improved pruritus among patients with severe atopic dermatitis [36]. It seems that anti-IL-31 antibody is likely to be efficient treatment for pruritic diseases, however its effectiveness in other diseases apart from AD needs further studies.
Research performed on transgenic mice has strongly suggested a potential role of IL-31 in AD and other inflammatory dermatoses with pruritus. Nowadays the prevailing view of the IL-31 role is pruritogenic cytokine. The IL-31 level has been shown to be elevated in plasma/serum in patients with AD [7, 8, 37, 38]. Moreover, IL-31 expression was increased in pruritic skin in AD compared with healthy individuals [7, 12, 39, 40]. Not only lesional skin but also non-lesional skin of AD patients expressed increased IL-31 mRNA levels [7]. Sonkoly et al. and Raap et al. showed that IL-31 serum levels in AD correlated with disease activity and severity [7, 8]. Similarly, in another study, Kim et al. demonstrated a correlation between serum IL-31 levels and the subjective itch intensity in AD patients [37]. Furthermore, serum IL-31 levels in AD correlated with cutaneous IL-31 mRNA, total serum IgE, eosinophil cationic protein and disease severity [33]. Contrary to these findings, Sokołowska-Wojdyło et al. reported no correlation between the IL-31 serum level and severity of pruritus in AD patients [38]. Neis et al. reported no correlation between the IL-31 mRNA level and both disease severity and IgE level in AD patients [39]. Nevertheless, a significantly higher expression of IL-31 in AD patients than in healthy individuals was observed in every single study suggesting its role in AD. Several studies showed an association of the IL-31 gene polymorphism with an increased risk of AD and non-atopic eczema [41, 42].
Moreover, several studies confirmed the role of IL-31 in AD in paediatric population, demonstrating a correlation between the IL-31 level and severity of AD [43, 44]. On the other hand, Raap et al. and Siniewicz-Luzeńczyk et al. revealed no correlation between serum IL-31 and itch intensity [43, 45]. Past reports strongly indicated IL-31 involvement in inducing both inflammation and pruritus in AD. Lack of correlation between IL-31 and pruritus might be explained by subjective evaluation of itch severity score. In conclusion, the serum IL-31 level is increased in AD compared to healthy subjects [37, 43–45]. The relationship between IL-31 and AD severity is less clear. In some studies [37, 43, 44, 46, 47], a positive correlation between IL-31 and AD severity was observed, while others showed no correlation [39, 45].
Observations of transgenic mice over-expressing IL-31, developing a skin phenotype closely mimicking atopic dermatitis [13] and increased levels of IL-31 in serum and skin of AD patients elicited research on the IL-31 role in the inflammation process. AD is mediated by T cells with a predominant Th2 type response and an increased number of cytokines IL-4, IL-5, IL-13 in acute skin lesions [48]. Interestingly, the IL-31 level correlates with the expression of IL-4 and IL-13. Thus, IL-31 was suggested to be involved in Th2-weighted inflammation diseases [14, 35, 49]. Nobbe et al. examined involvement of IL-31 in skin conditions according to the inflammatory profile and pruritus, namely T helper 1 cells (Th1) inflammation, Th2 inflammation and no inflammation [40]. Increased IL-31 immunoreactivity was observed only in AD compared with samples from other Th2-weighted diseases (MF, SS, alopecia areata), Th1-weighted diseases (psoriasis vulgaris, prurigo nodularis, psoriasis inversa) and conditions without inflammation (perilesional skin, pruritus sine materia, notalgia paresthetica) [40]. It was suggested that IL-31 is a mediator specific to atopic dermatitis rather than a pruritogen or general mediator in Th-weighted diseases. On the other hand, Sonkoly et al. revealed that lesional and non-lesional AD as well as prurigo nodularis skin expressed increased IL-31 mRNA levels [7].
IL-31 in the course of cutaneous T-cell lymphomas
Published data suggest that IL-31 with its receptor components (OSMR and IL-31 RA) are involved in AD and there are strong conditions for its involvement in inducing both pruritus and Th2-mediated inflammation. Furthermore, CTCL mimics AD when it comes to clinical presentation or laboratory findings (e.g. eosinophilia, increased IgE levels). Given this strong association, IL-31 was also investigated in CTCL. Key studies and findings are summarized in Table 1. There are only a few studies investigating the IL-31 role in CTCL due to the rarity of the disease. So far, only one study investigating IL-31 and its receptors expression in the CTCL skin was published [50]. Correlation of IL-31 levels in the skin and in blood of CTCL patients has not been studied yet. The studies were limited to investigation of IL-31 mRNA levels in peripheral blood mononuclear cells (PBMCs), levels of IL-31 in blood serum and the level of IL-31 and its receptors in the skin of CTCL patients and healthy individuals [50–54].
In addition to AD, CTCL was reported to overexpress IL-31 in serum and in the skin. Alike AD, patients with CTCL exhibit increased levels of serum IL-31 compared to healthy controls [51–54]. Moreover, Ohmatsu et al. reported a correlation between serum IL-31 levels and advanced CTCL disease stage, thus IL-31 is suggested to play a role in CTCL pathogenesis [53]. Singer et al. confirmed that IL-31 mRNA is increased in CTCL and also showed a significant correlation between IL-31 and patients experiencing marked pruritus [52]. Moreover, they reported a correlation between a decreased IL-31 serum level and resolution of pruritus in CTCL patients. No correlation between IL-31 and stage of the disease was seen, probably due to the fact that majority of patients were in advanced stages [52]. This is consistent with Mobs et al. findings, confirming lack of interrelation between the IL-31 level and CTCL stage [54]. Overexpressed IL-31 was noted in CTCL and was suggested to be involved in CTCL’s pruritus [52–54]. However, Malek et al. study did not confirm those findings, indicating that IL-31 is rather involved in CTCL pathogenesis [51]. There were no differences in the IL-31 level between pruritic and non-pruritic patients with CTCL, pruritus correlated with serum IL-31 concentration only in stage Ib [51]. Malek et al. research results do not match earlier findings and need further studies. On the other hand, Nattkemper et al. examined expression of IL-31 and its receptors (OSMR and IL-31RA) in the skin in CTCL patients [50]. IL-31 was significantly elevated in the epidermis and dermal infiltration, OSMR and IL-31RA were significantly elevated only in epidermis. Like in most of the previous research results, IL-31 level correlated with itch intensity [52, 54]. Similar to Singer et al. study results, the number of patients in early and advanced stages was rather small [50, 52]. It might be a possible reason for lack of correlation between IL-31 expression and disease stage. Nattkemper et al. confirmed these suggestions, indicating that IL-31 is involved in pruritus pathogenesis but it is irrelevant to pathogenesis of the disease itself [50].
Conclusions
Not only pathogenesis of CTCL but also pathophysiology of pruritus in CTCL remain unclear. It is likely that unknown proinflammatory agents produced in the microenvironment of the skin are involved, both in tumour growth and induction of pruritus in CTCL. The data presented in publications mentioned above have been the reason for continuous studies concerning the role of IL-31 in CTCL and pruritus. We already know that expression of IL-31 in serum in pruritic dermatoses including CTCL, according to some authors, is elevated. However, the question is whether overexpression of IL-31 in serum is associated with pruritus, disease itself or both. We also do not know whether IL-31 is directly associated with development of skin lesions in CTCL. Due to the great number of studies, IL-31 is best known for its pruritogenic role in AD, in contrast to its role in CTCL. IL-31 seems to play an important role in the signalling pathway involved in pruritus, moreover it is suggested to play a proinflammatory and immunomodulatory role. Undoubtedly, IL-31 is a promising cytokine, however its role in CTCL is not fully understood and it needs further studies.