Introduction
Local allergic rhinitis (LAR) is one of the endotypes of rhinitis [1]. The diagnosis was based on typical symptoms of allergic rhinitis associated with the suspected allergen, negative skin prick tests and allergen-specific IgE but positive result of nasal allergen provocation test [1].
The mechanism of LAR has similarities to allergic rhinitis. About 90% of LAR patients have nasal sIgE, but serum sIgE is not detectable. LAR is also associated with classical early and late allergic inflammation reactions [2, 3]. LAR individuals display nasal eosinophilic inflammation, and upon allergen exposure, there is a rapid increase and decrease in tryptase in the nasal secretions. In contrast, eosinophil cationic protein (ECP) increases progressively during 24 h.
Rondón et al. reported a group of 428 adult patients with rhinitis with a LAR prevalence of 25.7%, compared with 63.1% of allergic rhinitis (AR) and 11.2% of non-allergic rhinitis (NAR) [4]. The incidence of LAR ranges from 50% to 75% in a non-atopic patient with atopic nasal symptoms. The most frequently causative allergen in both forms was D. pteronyssinus.
Despite many studies analysing the prevalence, phenotype and mechanism of LAR, there are still many pending questions, for example, about the importance of this disease in children. Significant variation in the rate of LAR diagnoses (3.7–83.3%) in children and adolescents previously classified as having NAR [5] was observed. LAR is underdiagnosed and probably undertreated in those groups of patients.
Aim
The aim of the study was an attempt to assess the occurrence of LAR in Polish children with chronic rhinitis and to present its phenotype.
Material and methods
Study design
A cross-sectional observational study was undertaken to determine the prevalence and clinical characteristics of LAR in Polish children.
Patients
Patients were recruited in 8 allergy outpatient clinics representative of Poland’s central, southern and northern areas. Patients were recruited in comparable numbers from rural and urban centres. In each site, approximately 150 patients were pre-screened (according to medical history, medical data including computed tomography (CT) scan, results of endoscopy, and previous treatment). First, the medical bases of patients were analysed according to the diagnosis of rhinitis based on medical history and/or with the use of ICD-10 code (J30-J34). Then 50 participants from each centre were randomly selected and invited for diagnostic procedures.
The inclusion criteria were as follows:
aged 5 to 17,
gave consent to participate in the study (parents and children),
mild, moderate or severe persistent or intermittent rhinitis according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines.
The exclusion criteria were as follows:
clinical exacerbation of chronic rhinosinusitis or respiratory infections within 6 weeks before study initiation,
nasal polyposis, other serious diseases or chronic unstable disease,
nose deformity (anatomical changes in the nose that prevent nasal provocation, mainly mean nasal septum deviation).
Patient characteristics are presented in Table 1.
Table 1
Characteristics of the whole study group (n = 361)
Finally, 361 children were recruited out of 1184 pre-screened subjects. ENT doctors and allergologists performed the screening.
There were 176 women with a mean age of 9.5 ±5.3 years and 185 men with a mean age of 10.8 ±4.2 years. Characteristics of the group are presented in Table 1.
All the centres obtained permission to publish the data. All patients gave consent to participate in the study. The Bioethics Committee of the Medical University of Silesia approved the study.
Study protocol
Patients who met the inclusion criteria were selected to undergo further procedures, as follows: medical examinations, skin prick tests with inhalant allergens (SPT), serum total IgE and a full rhino-laryngological examination was performed using acoustic rhinometry and rhinoscopy, and in some patients, endoscopy and CT scans were performed.
Rhinitis was classified according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: rhinitis is considered persistent when symptoms are present for > 4 days/week or persist for > 4 consecutive weeks. Rhinitis severity was based on estimations of activity impairment (sleep, daily activities, work/school performance and troublesome behaviour) and was classified as severe, moderate or mild.
The severity of ocular and nasal symptoms, including obstruction, rhinorrhoea (watery, mucous, and purulent), itching, and sneezing, was recorded by a visual analogue scale (VAS) of 10 cm. Each symptom was categorized as ‘mild’ (VAS: 0–30 cm), ‘moderate’ (VAS: > 30–70 cm), or ‘severe’ (VAS > 70 cm).
Skin prick test (SPT)
The SPT was performed with a panel of aeroallergens: D. pteronyssinus, D. farinae, Phleum pratense, mugwort, birch, alder, hazel, Alternaria, Cladosporium, dog and cat epithelia (Allergopharma, Reinbek, Poland). A positive control (10 mg/ml histamine) and a negative control (saline) were included. An allergic reaction was defined as a positive skin test for at least one allergen, with a maximum wheal diameter of at least 3 mm or greater. Patients who did not exhibit a histamine reaction were excluded from further analyses.
Serum and specific IgE (sIgE)
Serum total and sIgE antibody levels to the same aeroallergens as used in the SPT panel were determined using a fluoroenzyme immunosorbent assay (UniCAP, Uppsala, Sweden). The positive cut-off value for sIgE levels was > 0.35 kU/l.
Nasal provocation test (NPT)
Nasal provocation tests were performed with acoustic rhinomanometry using an SRE 2000 rhinometry (Rhinometrics, Lynge, Denmark); these tests were performed according to the guidelines of the Standardisation Committee on Acoustic Rhinometry and EAACI position paper. The nasal provocation tests were performed when the concentrations of the examined allergens were lower in Poland. First, using a metered pump spray, the patients were intranasally challenged with saline to exclude nasal hyper-reactivity, and after it, NPT with extracts of D. pteronyssinus, D. farinae, alder, hazel, Phleum pratense, Alternaria, Cladosporium, mugwort, birch and cat (the concentration of each allergen was 5000 SBE/ml (Allergopharma, Reinbek, Germany). Additional NPTs were performed with one of the other extracts at 1-week intervals. The total volume of both nasal cavities was determined from 2–6 cm using acoustic rhinometry, and the results were compared with the baseline test. The immediate reaction was analysed at 15 min and 1 h – protocol based on the EAACI position paper.
The response was monitored at baseline, 15 min after saline application, and 15 min and 1 h after allergen application. The response was determined using a VAS and the total volume of the nasal cavity from 2–6 cm. An increase of ≥ 30% in the total VAS score and/or a decrease in total of ≥ 30% bilaterally in the 2–6 cm nasal volume were considered positive responses (in any of the analysed times after the challenge).
Patients were classified into three groups according to the clinical test results:
Statistical analysis
Statistica programme, version 8.0 (StatSoft, Poland) was used for all statistical analyses. A p-value < 0.05 was considered to be statistically significant. Descriptive analyses were performed with the mean and standard deviation. Non-parametric statistical analyses were performed using the χ2 test and multivariate ANOVA.
Results
As a result of the diagnostics carried out, the LAR was confirmed in 21% of patients, SAR in 43.9%, dual allergic rhinitis (DUAL) in 9.4% and NAR in 33.9% of patients. The details are presented in Table 1. The LAR group was dominated by girls, severe rhinitis and asthma were more common than other endotypes (p < 0.05).
Children with NAR were definitely more often exposed to passive smoking than other children (p < 0.05).
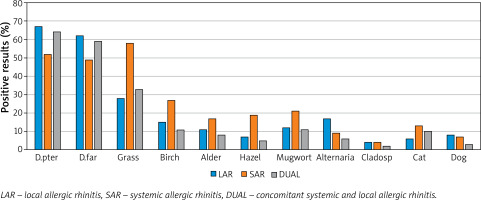
Based on NPT, allergy to HDM prevailed in the LAR group (68%), grass in the SAR group (58%), and the grass and HDM in the dual group (32% and 64%). Details are presented in Figure 1.
In the LAR group, apart from allergy to mites, the next most common were: hypersensitivity to grass (28%) and Alternaria (17%). In 13 (17%) patients, a reaction to every two allergens was observed – most often to house dust mites and grass in 7 cases, and to house dust mites and Alternaria in 3 cases. The frequency of allergy to mites was significantly higher in patients with LAR compared to SAR as well as to Alternaria (p < 0.05). There was no increase in the frequency of LAR with the age of the subjects (Table 2).
Table 2
Characteristics of study subgroups
| Parameter | LAR N = 76 | SAR N = 158 | DUAL N = 34 | NAR N = 122 |
|---|---|---|---|---|
| Age [years] (median) | 10.8 (7.2–14.2) | 10.2 (5.6–14.7) | 9.4 (7.8–15.9) | 10.6 (7.1–16.2) |
| Female | 48 (63%) | 81 (51%) | 16 (48%) | 74 (61%) |
| Severe rhinitis | 29 (38%)* | 33 (21%) | 11 (33%)* | 23 (19%) |
| Moderate rhinitis | 23(30%) | 60 (38%) | 13 (37%) | 44 (36%) |
| Mild rhinitis | 24 (32%) | 65 (41%) | 10 (30%) | 55 (45%)* |
| Intermittent | 29(38%) | 66 (42%) | 14 (41%) | 27 (22%)* |
| Persistent | 47 (62%) | 92 (58%) | 20 (59%) | 95 (78%)* |
| Positive family history of atopy | 19 (25%) | 107 (68%)* | 15 (43%) | 23 (19%) |
| Asthma | 27 (36%)* | 30 (19%) | 6 (18%) | 15 (12%) |
| AD | 2 (3%) | 13 (8%) | 2 (5%) | 1 (1%) |
| Urban area | 56 (74%) | 109 (69%) | 24 (71%) | 79 (65%) |
| Rhinitis in parents | 31 (41%) | 74 (47%) | 13 (38%) | 71 (58%) |
| Passive smoking | 22 (29%) | 6 (34%) | 9 (27%) | 50 (41%)* |
| Total IgE [IU/l] (median) | 18.6 (7.9–38.1) | 213.6 (116.9–246.1) | 174.8 (132–201) | 14.2 (8.9–56.8) |
Discussion
The occurrence of LAR in children and adolescents is relatively little studied. Several observational studies and meta-analyses emphasise the significant occurrence of this endotype of rhinitis in the youngest patients [5, 6]. In one study, authors found a 24.9 % incidence of LAR in patients with non-atopic rhinitis < 18 years of age. The obtained frequency of occurrence in the presented study is slightly higher, which may result from the influence of such factors as the study region, inclusion criteria, and false-positive or negative NPT results (estimated at 5–7% in this study). In the same meta-analysis, the occurrence of DAR was presented at 11.6% [6]. This discrepancy may be due to the difficulty in verifying the final diagnosis and the methods used to make the final diagnosis. In the presented study, negative results of tests and specific IgE but positive NPT for a given suspected allergen had to be in contrast to the allergy to the second allergen, for which all mentioned tests were positive. In the quoted analysis, we do not know the details of such diagnostics for the DAR. Compared to NAR, individuals with LAR reported more family history of atopy (62.8% vs. 16%) and more common exposure to house dust (60.5% vs. 12.9%). This was broadly consistent with our observations, with NAR patients testing negative and sIgE to all allergens, including house dust mites [5].
Certain limitations in the sensitivity and specificity of NPT, as well as the technique of their implementation, may affect the study’s final results by conducting different cohorts of patients. However, nasal challenge tests, preferably with several allergens, are highly recommended as diagnostic tools for LAR in children whenever possible [7].
NPT is the best way to diagnose LAR and can be further confirmed by finding a positive nasal-specific IgE and/or BAT result. A detailed history and examination of the nose should also be performed [8]. Unfortunately, such verification of NPT results is costly in studies with a large group of patients.
10-year observation of patients with LAR – increase in the incidence of severe nasal symptoms from 18.8% to 42% after 10 years – development of asthma in 12% of patients (suggesting a local allergic reaction of the bronchial mucosa in these patients) [9]. These data are consistent with the results of this study as patients with LAR more often had severe rhinitis and more often comorbid asthma compared to other endotypes. LAR should not be considered a benign disease as it has a natural tendency to worsen with significant impairment in quality of life [10].
An important feature distinguishing patients with LAR is the dominance of women (girls), which is confirmed by other authors [11].
However, LAR occurrence in children is still an underestimated and underdiagnosed problem. The study’s limitations were too small groups of patients, no nsIgE or BAT test, which would have introduced more restrictive LAR assessments, and no analysis of therapeutic interventions during the course of LAR. But such an observation is currently underway [12].