Adrenomedullin (AM) is a potent vasodilator peptide that was first identified in extracts of human pheochromocytoma [1]. To date, it is known to be synthesised in several organs and tissues, including the heart, lungs, kidneys, adipose tissue, and vascular endothelium [2]. AM affects the physiological functions of the cardiovascular system, kidneys, and central nervous system. It regulates blood pressure and vascular tone, increases cardiac output, and promotes diuresis and natriuresis [1–5].
Kitamura et al. [6, 7] were the first to report that two major molecular forms of AM circulate in the human blood, namely the biologically active mature AM (mature AM) form with an amidated C-terminus, and an inactive intermediate AM with a non-amidated C-terminal glycine. Although it is assumed that mature AM might be the key peptide with the AM function, circulating mature AM is quickly degraded and cleared from the circulation and is difficult to detect using a standard immunoassay because of the masking effect of its binding protein (complement factor H) [8]. The mid-regional AM (MR-proAM or pro-AM), which is the inactive fragment of AM, is more stable than mature AM and is used to predict the levels of mature AM [9]. In clinical settings, MR-proAM has been identified to be closely associated with the sequential organ failure assessment (SOFA) or acute physiology and chronic health evaluation (APACHE) II scores, and can be useful as a diagnostic and prognostic biomarker for sepsis [9–18].
Recently, a double monoclonal sandwich immunoassay was developed that can also measure bioactive mature AM, which is called bio-ADM [15, 19]. Several investigators have indicated that the levels of mature AM could serve as a useful and objective biomarker to predict severity, organ failure, and mortality in patients with sepsis [10, 20]. However, further evaluation is required for the clinical use of mature AM as a reliable biomarker of sepsis [8]. Therefore, in this study, we aimed to obtain additional information about mature AM, including its efficacy and the validation of the optimal cut-off, to help in its development as a marker of sepsis.
METHODS
Study protocol
This prospective, observational, single-centre study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Hospital Ethical Committee for Human Studies (Ref: 0-0317) on December 1, 2014, and retrospectively registered with the Japanese Clinical Trial Registry UMIN-CTR (Ref: UMIN000036474). General or written informed consent was obtained from all patients (age ≥ 20 years). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed in the preparation of this manuscript. For analysis, patients were enrolled upon admission to the intensive care unit (ICU) and diagnosed with sepsis or not according to the Sepsis-3 definitions, and then retrospectively categorised into non-sepsis or sepsis groups [21]. Exclusion criteria were age < 20 years, pregnancy, trauma, postoperative patients, or lack of informed consent.
Data collection
Clinical data obtained from electronic medical records included the first diagnosis, chronic comorbidities, laboratory test results, microbiology, and biomarker levels on admission to the ICU. Blood samples were collected for measuring the levels of AM peptides on ICU admission (day 1) and post-ICU admission day 3. The blood samples were collected into tubes containing 1.0 mg mL−1 EDTA-2Na and were split into small aliquots to avoid repeated freezing and thawing. The aliquoted samples were frozen as quickly as possible and stored at −70°C until use. AM is derived from an AM precursor via a two-step enzymatic reaction. First, a 185-amino acid AM precursor, known as preproadrenomedullin, is converted to glycine-extended AM, which is the 53-amino acid, inactive, intermediate form of AM (intermediate AM). Subsequently, by enzymatic amidation, intermediate AM is converted to mature AM, a 52-amino acid peptide with an amide group at the C-terminus [6, 7]. Because conventional assays cannot distinguish between intermediate and mature AM forms, AM sometimes indicates total AM, which is the sum of the levels of intermediate and mature forms. In our study, plasma levels of both mature and total AM were measured by a specific fluorescence immunoassay (Tosoh Corporation, Tokyo, Japan) using two independent antibodies – one that binds to the ringed structure was used for the total AM assay, and the other that binds to the middle region between the ring and the C-terminal portion of the peptide was used for the mature AM assay, as described previously [22, 23]. Plasma presepsin (PSEP) levels were measured with a rapid chemiluminescent enzyme immunoassay (CLEIA) using a PATHFAST immunoanalyser (Mitsubishi Chemical Medience, Tokyo, Japan). Additionally, we obtained data on procalcitonin (PCT), white blood cells (WBCs), C-reactive protein (CRP), lactate, platelets, and creatinine, which were clinically measured in serum samples at the central laboratory of the University of Miyazaki Hospital.
Outcomes
The primary outcome of the study was the diagnostic performance of biomarkers and SOFA score on ICU admission for sepsis. Secondary outcomes were the time course of AM levels and the prognostic performance of biomarkers and SOFA score for sepsis.
Statistical analysis
Statistical analysis was performed using Med-Calc 17 (MedCalc Software Bvba, Ostend, Belgium) or JMP 15 (SAS Institute Inc., Cary, NC, USA). Data are expressed as median (IQR: interquartile range) or exact numbers. Categorical data were examined using the chi-square or Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U or Kruskal-Wallis test, as appropriate. Pearson product-moment correlation coefficient was used to measure the strength of the relationship between two variables. Receiver operating characteristic (ROC) analysis and comparison of the area under the curve (AUC) were performed to evaluate the accuracy of the biomarkers in diagnosing and predicting sepsis. A value of P < 0.01 was considered statistically significant.
RESULTS
Diagnosis of sepsis on ICU admission
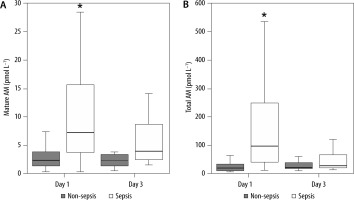
Ninety-eight patients were enrolled in this study between January 2015 and November 2018 (Table 1). A total of 56 patients were diagnosed with sepsis according to the Sepsis-3 definitions, and 42 were not diagnosed with sepsis. The abdomen and respiratory tract were the most common sites of infection in patients with sepsis. The most common reason for admitting patients with no sepsis to the ICU was heart failure, followed by acute pancreatitis, gastrointestinal bleeding, aortic disorders, interstitial pneumonia, and autoimmune diseases, among others. We could not track the outcome for one patient with sepsis who was transferred to another hospital. The levels of AM peptides, values of clinical parameters including laboratory results, and SOFA score on ICU admission are presented in Table 2. The levels of mature AM, total AM, PCT, and PSEP were substantially higher in patients with sepsis than in those without sepsis on day 1 (Figure 1 and Table 2). However, these significant differences in the levels of both AM peptides disappeared on day 3 (Figure 1).
TABLE 1
Clinical characteristics of patients admitted to ICU by Sepsis-3 classification
| Factor | Non-sepsis (n = 42) | Sepsis (n = 56) | P-value |
|---|---|---|---|
| Age (years), median (IQR) | 66.5 (56.0–76.0) | 69.0 (58.5–78.0) | 0.48 |
| Male, n (%) | 32 (76) | 37 (66) | 0.27 |
| Diagnosis, n (%) | |||
| Abdominal disorder | 8 (19) | 16 (29) | < 0.01 |
| Abscess | 1 (2) | 2 (4) | |
| Aortic disorder | 3 (7) | 0 (0) | |
| Blood stream infection | 0 (0) | 8 (14) | |
| Heart failure | 10 (24) | 0 (0) | |
| Neurological disorder | 1 (2) | 2 (4) | |
| Respiratory disorder | 8 (19) | 15 (27) | |
| Skin/Soft tissue disorder | 3 (7) | 4 (7) | |
| Urinary disorder | 3 (7) | 5 (9) | |
| Others | 5 (12) | 4 (7) | |
| 28-day mortality, n (%) | 6 (14) | 17 (31*) | 0.05 |
TABLE 2
Clinical parameters of patients on ICU admission (day 1)
FIGURE 1
Comparative plasma levels of mature and total adrenomedullin (AM) between patients with and without sepsis. Group comparisons were performed using Kruskal-Wallis and post-hoc Mann-Whitney U tests. *P < 0.01
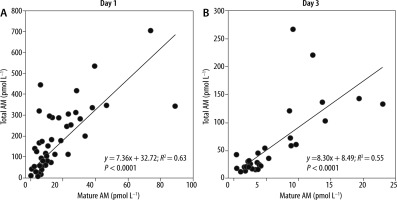
To compare the diagnostic accuracy of sepsis using each biomarker, we performed an ROC analysis and determined the AUCs using the values obtained for the 98 patients on ICU admission (Table 3). The AUCs of mature and total AM were 0.848 and 0.879, respectively, indicating that both AM peptides exhibit significant predictive values for the diagnosis of sepsis, in comparison with other biomarkers, such as PCT, PSEP, SOFA score, and lactate levels (0.828, 0.682, 0.772, and 0.660, respectively). The cut-off levels of mature and total AM for the diagnosis of sepsis were 5.2 pmol L−1 (≈ 31.2 pg mL−1) and 48.3 pmol L−1 (≈ 289.8 pg mL−1), respectively (Table 3). Significant correlations between the levels of mature and total AM were found in blood samples obtained from the same patients on days 1 and 3 (Figure 2).
TABLE 3
Diagnostic performance of different clinical parameters on ICU admission (day 1) for sepsis
FIGURE 2
Relationships between mature and total adrenomedullin (AM) in non-sepsis and sepsis patients on admission to the intensive care unit (day 1) and on day 3. The Pearson product-moment correlation coefficient was used to measure the strength of the relationship between two variables
The 28-day mortality rates in patients without or with sepsis were 14.3% and 30.9%, respectively (Table 1). In patients with sepsis, the levels of mature and total AM were not statistically significant on day 1; however, they were significantly increased in non-survivors on day 3 compared with the levels in survivors (Figure 3). Likewise, the AUCs of these AM peptides for predicting the 28-day mortality rates in patients with sepsis became significant on day 3, but not on day 1 (Table 4). The cut-off levels of mature and total AM for the 28-day mortality on day 3 were 5.4 pmol L−1 (≈ 32.4 pg mL−1) and 72.5 pmol L−1 (≈ 435.0 pg mL−1), respectively (Table 4).
FIGURE 3
Comparative plasma levels of mature and total adrenomedullin (AM) between survivor and non-survivor patients with sepsis. Group comparisons were performed using Kruskal-Wallis and post-hoc Mann-Whitney U tests. *P < 0.01
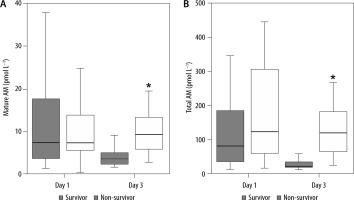
TABLE 4
Prognostic performance (predicting 28-day mortality) of adrenomedullin (AM) in septic patients
DISCUSSION
In this study, we found that the plasma levels of mature and total AM on day 1 of ICU admission in patients with sepsis were significantly higher than those in patients without sepsis. The good correlation between the plasma levels of both peptides observed in the present study demonstrates that both total and mature AM are equally useful in diagnosing sepsis based on the latest Sepsis-3 definitions. In contrast, with regards to the clinical outcome in patients with sepsis, a significant difference in the level of each AM was found between survivors and non-survivors 3 days after admission. In addition, the levels of both AM forms could predict the 28-day mortality in patients with sepsis on day 3, but not on day 1. Therefore, unlike in previous studies, we found that it may take several days for these AM peptides to prognosticate the 28-day mortality in patients with sepsis because AM levels increased even in patients with a good prognosis on day 1.
In some previous studies, the levels of total AM were assessed and their utility in diagnosing sepsis was compared with that of other chemical biomarkers, such as CRP, PCT, lactate, and PSEP [8, 9, 18, 20, 24–26]. We found that among all the clinical parameters examined, the diagnostic accuracy for sepsis on the first day of ICU admission was the highest for total AM, and that of mature AM was the second highest. It is assumed that mature AM is biologically active, and would thus be a more informative biomarker than total AM in patients with sepsis. In our study, the cut-off level of mature AM for the diagnosis of sepsis was approximately 31.2 pg mL−1, and its AUC was 0.848, with a sensitivity of 88.1% and a specificity of 67.9%. Other investigators have also reported that the ability of mature AM to identify patients with sepsis was modest because an AUC of 0.76 and a Youden’s index derived cut-off of 37 pg mL−1 generated a sensitivity of 61% and a specificity of 80% [20]. These cut-off values of mature AM were similar, indicating that values of about 30 pg mL−1 could be applicable to diagnose sepsis, which is a novel finding on mature AM.
The half-life of mature AM in plasma is approximately 20 min. As a biomarker of acute cardiovascular failure, the levels of mature AM can change significantly during the first 24 h after diagnosis [10]. Indeed, in our study, the levels of mature AM in patients with sepsis significantly increased on day 1 but reverted to the same levels as in patients without sepsis 3 days after admission, indicating that the time frame in which sepsis can be identified by this peptide may be confined to an early stage after the onset.
Accumulating evidence indicates that clinical outcome in patients with sepsis can be determined based on AM levels [10–12, 14–17]. However, investigators have pointed to the need for more data to evaluate the mortality rate in patients with sepsis prognosticated by the levels of mature AM [8]. Marino et al. [15] found that the levels of mature AM at ICU admission strongly correlated with the 28-day mortality rate in patients with sepsis. Patients with AM levels > 70 ng L−1 had a 28-day survival rate of 55%. Recent evidence supports this result. Kim et al. [10] reported that the cut-off value of mature AM levels for 28-day mortality was 97.12 pg mL−1. However, in the present study, the levels of total and mature AM on day 1 could not be used to predict the 28-day sepsis-related mortality. It is well known that AM levels are elevated in patients with heart failure, myocardial infarction, pulmonary hypertension, SIRS, inflammatory bowel disease, and renal failure, but this does not necessarily correlate with prognosis [2, 27–30]. Therefore, even patients with sepsis who have a good prognosis may have elevated AM levels early in the course of sepsis. This may explain the results presented in Figure 3, showing no significant difference in the levels of mature and total AM between non-survivors and survivors on ICU admission (day 1) when multidisciplinary treatment had not yet been initiated.
We found that the levels of mature and total AM on day 3 in patients with sepsis had a high predictive value for sepsis-related mortality as determined by ROC analysis. Interestingly, Mebazaa et al. [11] reported that in patients with mature AM levels > 70 pg mL−1 on admission, a decrease in the levels below 70 pg mL−1 on day 2 was associated with the recovery of organ function on day 7 and a better 28-day outcome (9.5% mortality), whereas persistently elevated levels of mature AM on day 2 were associated with prolonged organ dysfunction and high 28-day mortality (38.1% mortality). These results suggest that the levels of mature AM may remain high if treatment is unsuccessful and organ damage continues in sepsis. Indeed, as demonstrated in Figure 3, the sustained increase in the levels of mature AM in patients with sepsis correlated with worsening prognosis.
In the present study, the cut-off levels of mature and total AM on day 3 for the diagnosis of the 28-day sepsis-related mortality were approximately 32.4 and 435.0 pg mL−1, respectively. The cut-off level of mature AM was lower than that in previous studies (30 pg mL−1 vs. 70 pg mL−1). This could be explained by the sensitivity of the assay used in each study, indicating that the cut-off values obtained by different assay methods may be different. Recently, Lundberg et al. [20] reported a Youden’s index cut-off of 45 pg mL−1 for predicting the 28-day sepsis-related mortality in their sepsis cohort, which was as low as that in the present study.
It has been presumed that total AM represents the sum of synthesised intermediate AM and mature AM. However, little is known about the ratio of each peptide in total AM. We suspected that if the composition ratio varied for each patient with sepsis, the levels of total AM might not directly reflect the functional status of mature AM [31]. However, as discussed previously, we found a definite correlation between the levels of mature and total AM, both of which were measured from an identical blood sample, with a resultant increase in a similar fashion. Based on our results, it may be sufficient to measure either mature or total AM for the diagnosis of sepsis.
LIMITATIONS OF THE STUDY
This study has some limitations. First, this study was performed as a small, single-centre study focusing on the diagnostic performance of AM for sepsis and septic shock based on the Sepsis-3 definitions. Given the similarities in responses and outcomes, further studies with a much larger patient sample size and multi-centre design are required. Second, as discussed previously, the AM-binding protein, complement factor H, hinders the reliable measurement of mature AM [9, 18, 32]; this technical difficulty in the assay precludes its practical use. Recently, Weber et al. [19] reported a new assay to reliably measure the bioactive AM using a double monoclonal sandwich immunoassay that can measure C-terminally amidated biologically active AM. We used our originally developed assay for measuring mature AM and found that the limits of detection and quantitation were 0.133 and 0.085 pmol L−1, respectively, according to the Clinical and Laboratory Standards Institute (CLSI) protocols. The intra- and inter-assay coefficients of variation were 1.8% and 5.1%, respectively [23]. Thus, we believe that our assay is as reliable as the one reported by Weber et al. Third, we do not provide any evidence of whether AM might resolve or worsen the septic state itself. Although the effects of AM or AM antibody therapy for sepsis have been evaluated in some preclinical animal studies [33], it is still unclear whether AM will have beneficial therapeutic effects in patients with sepsis. There is an ongoing, multi-centre study evaluating AM-binding antibody therapy for sepsis (AdrenOSS-2) [34]. The results of this study might eventually be useful in characterising the efficacy of AM therapy in patients with sepsis.
CONCLUSIONS
We conclude that (1) at the early onset of sepsis, both mature and total AM are excellent biomarkers with the highest diagnostic accuracy for sepsis among the biomarkers that we evaluated; (2) a good correlation exists between each AM, indicating that the changes in the plasma levels may directly reflect one another; and (3) both AM peptides have the potential to predict the 28-day mortality in patients with sepsis several days after the onset. Further studies are required to clarify the relationship between mature AM and its clinical significance in comparison with total AM.




