Intra-abdominal hypertension (IAH) is defined as a repeated or sustained intra-abdominal pressure (IAP) above or equal to 12 mm Hg, and abdominal compartment syndrome (ACS) is defined as a sustained IAP above 20 mm Hg associated with new organ dysfunction or failure [1].
A previous case report described how non-invasive ventilation (NIV) caused aerophagia, gastric distension and increased IAP, culminating in ACS and cardio-respiratory arrest. Resuscitative efforts included gastric decompression with a nasogastric tube, which resulted in instant clinical recovery, improved oxygen requirements, tidal volumes and IAP [2]. NIV is a common treatment in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD). Its indications have extended to various other disease processes where it has proven its value. NIV has limitations, and many factors can cause NIV failure. One factor is a rising PaCO2, requiring invasive mechanical ventilation. NIV can increase IAP by gas insufflation into the stomach, especially in patients with an altered mental status. As a result of this increase in IAP, elevation of the diaphragm may occur, causing atelectasis and increased PaCO2 via reduced tidal volumes.
This study’s main aim was to determine whether the use of NIV increases IAP and whether raised IAP is associated with failure of NIV.
METHODS
Ethical regulations
This prospective multi-centre observational trial was conducted in accordance with the study protocol, the Declaration of Helsinki and applicable regulatory requirements. The Ethics Committee of the South Metropolitan Health Service in Perth, Australia approved the study (13/20). Subsequently, institutional ethics committees for each site approved the study. Informed consent was obtained from all patients before enrolment. Only deidentified patient data were collected. The participating study sites were recruited via the Clinical Trials Working Group of the Abdominal Compartment Society (formerly known as the World Society of Abdominal Compartment Syndrome, www.wsacs.org). The study was also approved and registered as an official Abdominal Compartment Society study (No. 14, www.wsacs.org).
Patient selection
Inclusion criteria were patients admitted to intensive care, requiring NIV (defined as bilevel positive airway pressure with alternating inspira-tory and expiratory supportive pressures), signed informed consent, aged over 18 years, and requiring a urinary catheter as part of the patient’s routine management. Patients were excluded if they were pregnant, had a contraindication to IAP monitoring, were moribund and unlikely to survive the first 24 hours or if they were ineligible for intensive care without restrictions or limitations.
Protocol and data collection
IAP measurement and blood gas analysis were performed before the application of NIV. After NIV was started, a set of measurements were performed every 4 hours for the first 48 hours, then daily until the patient was discharged. All patients were orientated and cooperative when started on NIV. The IAP was measured via the standard bladder technique as recommended by the Abdominal Compartment Society [1]. A nasogastric tube was not routinely inserted in the study population.
In addition to patient demographics, the severity of illness during the 24-hour period before the first IAP measurement was documented using the Acute Physiology and Chronic Health Evaluation II score [2] and the Sequential Organ Failure Assessment II score [3].
Statistical analysis
The computer packages IBM SPSS Statistics for Mac, version 26.0 (IBM, St Leonards NSW, Australia) was used for statistical analysis. Data are expressed as mean with standard deviation (±SD) in the case of normal distribution or as median (interquartile range) for non-normally distributed parameters. The Shapiro-Wilks test was used to test normality. To analyse statistical significance (P < 0.05), we used the Wilcoxon signed-rank test to compare within a group or subgroup and the Mann-Whitney U test to compare between subgroups.
RESULTS
One patient was excluded as IAP was not measured after the application of NIV. The 35 included patients were predominantly male (n = 25, 71.4%) with a mean (±SD) age of 67.8 (±12.5) years, median (interquartile range) body mass of 79 (70–95) kg, height of 1.71 (±0.09) m, body mass index of 27.9 (24.5–30.0) kg m–2, Acute Physiology and Chronic Health Evaluation II score of 15.8 (±6.4), Simplified Acute Physiology II Score of 38.1 (±12.9), and Sequential Organ Failure Assessment score of 6 (5–9).
Thirty-five patients required NIV for at least 24 hours, 26 patients required NIV for at least 48 hours and 8 patients required NIV for at least 72 hours. No patient required NIV beyond 72 hours.
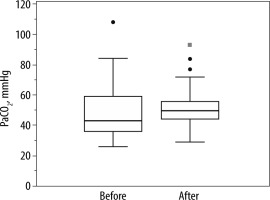
On admission and after 24 hours of NIV IAP was 11.0 (7.5–15.0) mm Hg and 11.0 (8.5–14.5) mm Hg (P = 0.82) and PaCO2 was 44.4 (±11.4) mm Hg and 51.3 (±14.3) mm Hg (P = 0.19), respectively (Figures 1 and 2).
FIGURE 1
Intra-abdominal pressure (IAP) of 35 patients at baseline (before) and after 24 hours (after) of non-invasive ventilation. IAP did not change over this period

FIGURE 2
Arterial partial pressure of carbon dioxide (PaCO2) of 26 patients at baseline (before) and after 24 hours (after) of non-invasive ventilation. PaCO2 did not change over this period
Twenty-three (65.7%) patients required pressure support > 10 cmH2O, and 12 (34.3%) required pressure support ≤ 10 cmH2O. IAP and PaCO2 did not significantly differ between the subgroups based on their required pressure support (P = 0.18 and P = 0.40, respectively). IAP did not increase following 24 hours of NIV in both subgroups (pressure support > 10 cmH2O: 13.0 [9.0–15.0] mm Hg to 12.0 [11.0–16.0] mm Hg, P = 0.69 and pressure support ≤ 10 cm H2O: 9.0 [7.0–12.5] mm Hg to 9.2 [6.3–13.8] mm Hg, P = 0.96, respectively). Equally, following 24 hours of NIV, the PaCO2 did not significantly increase, either in the patients requiring pressure support > 10 cm H2O (37 [36–55] to 53 [45–62] mm Hg, P = 0.14), or in those with pressure support ≤ 10 cm H2O (46 [36–65] to 49 [37–53] mm Hg, P = 0.37, respectively).
There were 15 (43%) patients with IAH on admission and after 24 hours of NIV. The PaCO2 did not differ between the groups before (P = 0.89) or after 24 hours of NIV (P = 0.25). IAP did not change in the patients who received NIV for 48 hours (P = 0.75) and 72 hours (P = 0.78).
DISCUSSION
The polycompartment syndrome has been described as the interactions between compartmental pressures of different body compartments [4]. The use of invasive ventilation and the use of positive end-expiratory pressure have been associated with increases in IAP [5–8]. Little is known about the impact of NIV on IAP. To the best of our knowledge, only one case report has been published previously [9]. One might suspect that the application of NIV may be associated with increases in IAP because of voluntary muscle contractions and accessory muscle use during forced expiration in patients with chronic obstructive pulmonary disease or due to aerophagia.
The present study could not demonstrate an impact of NIV even in the patients requiring pressure support above 10 cmH2O on IAP. We also did not observe any adverse effect on PaCO2, regardless of IAP and the presence of IAH. Our findings suggest that patients with IAH tolerate NIV, and IAH should not be considered a contraindication to initiate NIV. We suggest the placement of a nasogastric tube in addition to IAP monitoring in patients who require NIV and are at risk of developing IAH.
A recent review found the application of NIV to be safe in patients with acute respiratory failure after abdominal surgery [10]. However, in this review, IAP was not assessed. Aerophagia can occur in up to half of patients with NIV and may lead to discontinuation. The use of gastroprokinetic drugs or changes in NIV settings may help [11]. However, if abdominal distension due to NIV persists or repeatedly reoccurs, further investigations to rule out any co-existing underlying abdominal pathology have been recommended [11]. The normal esophago-gastric sphincter pressure is thought to be above 10 cm H2O and reduced in about half of the patients with gastro-oesophageal reflux disease [12, 13]. However, applying pressure support of more than 10 cm H2O did not influence IAP in our cohort. It is thought that patients can to some degree learn to handle and eliminate gas effectively [11].
The study has several limitations. First, the sample size was small. Second, our observation period was limited to 24, 48, and 72 hours in 35, 26 and 8 patients, respectively. Third, we did not assess whether applying pressure support above the patient’s IAP causes gastric distention. Fourth, we did not collect extensive demographic data including the presence of gastro-oesophageal reflux disease. And last, we recognize the difficulty of measuring IAP in awake patients. We cannot rule out that some IAP readings were influenced by abdominal muscle contraction during forced expiration in patients requiring NIV.
CONCLUSIONS
NIV was not associated with an increase in IAP over 72 hours in this small observational study, regardless of whether the pressure support applied was greater or smaller than 10 cmH20. Patients with IAH appeared to tolerate NIV, and IAH should not be considered a contraindication to initiate NIV.




