Introduction
Periodontitis is a chronic multifactorial inflammatory disease characterized by the progressive destruction of periodontium, primarily associated with dental biofilm. Clinically, periodontitis presents symptoms such as increased probing pocket depth, gingival bleeding, radiographic bone loss, clinical attachment loss, and tooth loss [1]. Gingivitis, linked to dental biofilm and/or endogenous hormonal fluctuations, medications, systemic diseases, and inadequate nutrition, exhibits fundamental features, including swelling, edema, redness, and bleeding of the gums. Gingivitis is considered a prerequisite for the development of periodontitis [2]. Healthy periodontium is defined by the absence or minimal presence of inflammatory signs in reduced or early periodontium [3]. Early diagnosis of gingivitis and periodontitis is crucial for effective management of periodontal diseases. Utilizing specific mediators present in gingival crevicular fluid (GCF) can aid in the early detection of these conditions [4].
Interleukin 1β (IL-1β) is a pro-inflammatory cytokine belonging to the interleukin 1 family [5]. It serves as a crucial mediator in the inflammatory response, primarily inducing the production of other cytokines. Associated with various cellular activities such as cell proliferation, differentiation, and apoptosis, IL-1β exerts biological effects that include the stimulation of T-lymphocytes and lymphokine production, proliferation of B-lymphocytes and antibody production, fibroblast proliferation, stimulation of prostaglandin (PGE2) release by monocytes and fibroblasts, and the secretion of metalloproteinases that degrade extracellular matrix proteins. Interleukin 1β facilitates osteoclast formation and bone resorption, and influences neutrophil chemotaxis and activation [6]. The role of IL-1β in periodontal disease has been the subject of numerous studies in the past. One study suggested that much of the damage occurring during periodontal tissue destruction could be associated with the activity of IL-1 and tumor necrosis factor α (TNF-α) [5]. Another study found that IL-1β is more effective in bone resorption compared to cytokines such as IL-1A, TNF-α, and lymphotoxin [7]. Interleukin 1β has been identified to play a leading role in the pathogenic mechanism of periodontal tissue destruction, and the severity of periodontal disease can be assessed based on the activity of this cytokine. Researchers suggest that clinical parameters such as gingival index (GI), probing pocket depth (PPD) and GCF IL-1β activity are correlated. The inflammatory degree in periodontally affected tissues can be measured through the activity of IL-1β in the GCF [5, 8, 9].
Nesfatin-1, discovered in 2006, is a peptide responsible for appetite control and secreted from the hypothalamic nuclei [10]. Derived from nucleobindin 2 (NUCB2), nesfatin-1 plays a significant role in appetite regulation. This peptide is found not only in the central nervous system (CNS) but also in peripheral tissues; specifically, it is secreted by peripheral adipose tissue, gastric mucosa, pancreatic endocrine β cells, and testicular tissue. Among the physiological effects of nesfatin-1, it plays a crucial role in anorexic effects, food intake, regulation of blood sugar, cardiac function, water intake, gastric emptying, stress responses, and anxiety control. In addition to the various effects of nesfatin-1, there is an important aspect not yet fully understood but supported by limited literature: its potential anti-inflammatory activity. Studies conducted on rat brain tissues have shown that nesfatin-1 exhibits anti-inflammatory and anti-apoptotic effects. Furthermore, it has been observed that nesfatin-1 reduces the concentrations of TNF-α, IL-1β, and IL-6 and also it has been found to suppress IL-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes, potentially through the NF-κB, MAPK, and Bax/Bcl-2 pathways [11]. These findings contribute significantly to the understanding of nesfatin-1’s potential anti-inflammatory properties [12, 13].
Apart from its physiological effects on appetite and metabolic regulation, nesfatin-1 exhibits potential anti-inflammatory properties, as evidenced by its ability to reduce the levels of pro-inflammatory cytokines such as IL-1β and TNF-α. These findings suggest a possible association between nesfatin-1 and inflammatory processes in periodontal diseases, warranting further investigation. Furthermore, we hypothesize that nesfatin-1 levels will exhibit a significant decrease in patients with periodontitis. This study aims to understand the potential roles of IL-1β and nesfatin-1 in the pathogenesis of periodontal diseases by examining their levels among periodontitis, gingivitis, and healthy control groups. Furthermore, the present study aims to investigate the potential relationship between nesfatin-1 and the pro-inflammatory effects of IL-1β, as well as whether these mediators correlate with clinical parameters.
Material and methods
Study design
Our research, conducted within the Department of Periodontology at Kahramanmaraş Sütçü İmam University Faculty of Dentistry, enlisted a diverse cohort of 62 participants, including 31 men and 31 women, spanning ages 20 to 60. After providing comprehensive information about the study’s objectives and methodology, we obtained signed voluntary consent from each participant. Ethical clearance was diligently secured from the Kahramanmaraş Sütçü İmam University Local Ethics Committee, with approval granted on September 14, 2021, under decision number 203.
Inclusion criteria
Individuals meeting the following criteria were included in our study: those who were systemically healthy, not pregnant or in the lactation period, had not undergone periodontal treatment within the last 6 months, and had not used antibiotics or oral contraceptives in the last 6 months. Participants were also required to have a minimum of 20 teeth, excluding third molars.
In determining the study groups, we followed the 2017 Classification Criteria for Periodontal and Peri-implant Diseases and Conditions. The healthy group was characterized by a probing depth < 3 mm, no radiological bone loss, and bleeding on probing (BOP) < 10%. The gingivitis group exhibited a probing depth < 3 mm, no radiological bone loss, and BOP > 10%. The periodontitis group included individuals with interdental clinical attachment loss of ≥ 2 mm in at least 2 non-adjacent teeth, buccal or lingual probing depth ≥ 3 mm in 2 or more teeth, and clinical attachment loss ≥ 3 mm. All patients in the periodontally healthy and gingivitis groups had an intact periodontium.
Clinical measurements
Before collecting GCF samples, clinical measurements were conducted to assess patients’ periodontal condition. The examination included measurement of the plaque index (PI) [14], PPD, GI [15], clinical attachment level (CAL), and BOP. Plaque index, GI, and BOP were measured on four surfaces of each tooth, while PD and CAL were measured on the six surfaces beneath each tooth.
Gingival crevicular fluid collection
For the periodontitis group, GCF samples were collected from the deepest pocket area based on the patient’s radiological examination. Sterile paper strips were used to collect GCF samples. After isolating the areas with cotton swabs, plaque was removed with the aid of a probe. The region was carefully dried with an air spray in both the vestibular and palatal areas perpendicular to the long axis of the tooth. Salivary contamination was prevented. Subsequently, paper strips were placed into the sulcus. Each paper strip was gently placed into the gingival sulcus until slight resistance was felt (Brill’s technique). After being left in the sulcus for 30 seconds, the strips were placed in the previously calibrated Periotron 8000 device to measure and record the volumes. The obtained values were converted to microliters (µl), and the GCF volume was calculated. This conversion process involved sending the Periotron 8000 measurement values to a computer with the PERIOTRON program via serial connection. To prevent potential liquid contamination after each volume determination, the poles of the device were wiped with a dry gauze. Samples contaminated with blood and saliva were not included in the evaluation. Paper strips containing GCF samples were placed in Eppendorf tubes and stored at –80°C until the day of analysis.
Biochemical analysis
Gingival crevicular fluid samples were transferred to Eppendorf tubes, and 500 µl of physiological saline was added to each sample. The samples were left overnight at +4°C. After the incubation period, Eppendorf tubes were centrifuged, and the supernatant was separated. Nesfatin-1 and IL-1β levels in the samples were measured using the enzyme-linked immunosorbent assay (ELISA) method.
Statistical analysis
In order to detect a meaningful change in nesfatin-1 and IL-1β levels among groups with periodontal diseases, a minimum of 19 subjects per group was determined, ensuring a change of 1.4 ±1.5 units (effect size = 7.6-9) to be statistically significant (α = 0.05, 1-β = 0.80). Jamovi software (Version 2.3.21) was used for statistical analysis. Data are presented as mean ± standard deviation. The normality of distribution was checked with the Kolmogorov- Smirnov test. Due to the normal distribution, ANCOVA analysis was conducted to detect the differences between groups and age was added as a covariate. The Tukey post-hoc test was conducted for multiple comparisons analysis. Gender differences between groups were calculated using a chi-squared test. The relationship between periodontal and inflammatory variables was analyzed with the Pearson correlation test. A value of p < 0.05 was accepted as statistically significant.
Results
When examined in terms of oral hygiene habits, significant differences were found among the groups (p < 0.05). Particularly in the age range of 40-60, the number of cases of periodontitis was observed to be higher compared to the gingivitis and healthy groups. The frequency of tooth brushing in the healthy group was found to be significantly lower than that in the gingivitis and periodontitis groups (p < 0.05). However, no significant difference was detected in terms of gender and education (p > 0.05) (Table 1).
Table 1
Demographic attributes of the subjects included in the study according to case-control groups
All oral PI, GI, PPD, and BOP indices showed statistically significant differences among the healthy, periodontitis, and gingivitis groups (p < 0.05). The CAL index in the entire mouth showed no significant difference between the gingivitis and healthy groups, while it demonstrated a significant difference between the periodontitis group and the gingivitis and healthy groups (p < 0.05) (Table 2).
Table 2
Relationship between oral health indices and case-control groups, median (min-max)
1 ANCOVA (age was added as a covariate) and Tukey post hoc test. Different superscripts indicate a significant difference between groups (p < 0.05)
PI – full mouth plaque index, GI – full mouth gingival index, PPD – full mouth periodontal pocket depth, BOP – full mouth bleeding on probing, T-PI – sampled teeth plaque index, T-GI – sampled teeth gingival index, T-PPD – sampled teeth periodontal pocket depth, T-BOP – sampled teeth bleeding on probing, MT – missing teeth, GCF – gingival crevicular fluid, T-CAL – sampled teeth clinical attachment level
For the sampled region, statistically significant differences in PI, GI, PPD, and BOP indices were found among the healthy, periodontitis, and gingivitis groups (p < 0.05). The volume of GCF showed statistically significant differences among the healthy, periodontitis, and gingivitis groups (p < 0.05) (Table 2).
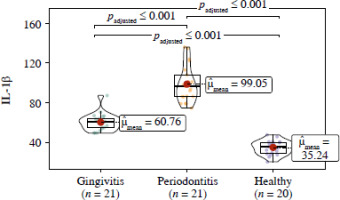
When examining the IL-1β value, a significant difference was found among the healthy, gingivitis, and periodontitis groups (p < 0.001), with the highest IL-1β value measured in the periodontitis group (Fig. 1).
Fig. 1
Violin plot presenting the relationship between IL-1β and case-control groups (ANCOVA and Tukey post hoc test, age was added as a covariate)
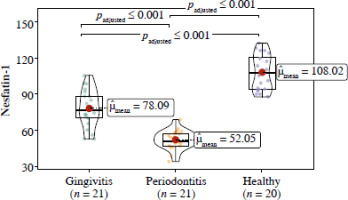
Regarding the nesfatin-1 value, a significant difference was found among the healthy, gingivitis, and periodontitis groups (p < 0.001), with the lowest nesfatin-1 value measured in the periodontitis group (Fig. 2).
Fig. 2
Violin plot illustrating the correlation between nesfatin-1 and case-control groups (ANCOVA and Tukey post hoc test, age was added as a covariate)
A significant positive correlation was found between IL-1β and all oral indices (p < 0.05). Similarly, nesfatin-1 showed a significant positive correlation with all oral indices (p < 0.05) (Fig. 3).
Fig. 3
Correlation between nesfatin-1 and IL-1β with clinical data. Correlation matrix boxes: positive correlations are represented by green boxes, while negative correlations are indicated by orange boxes (color tones signify the strength of the correlation). Boxes lacking an ‘X’ signify significance (p < 0.05). The numbers on the boxes are Pearson correlation coefficient values

Discussion
Periodontal diseases, with a primary focus on periodontitis, have undergone extensive research efforts, given their multifactorial nature and significant impact on oral health. In the present study, our objective was to investigate the potential roles played by two pivotal biomarkers, IL-1β and nesfatin-1, in the intricate pathogenesis of periodontal diseases. Through our findings, we illuminate the complex interplay between inflammatory mediators and systemic factors within the broader context of periodontal health.
Characterized by a chronic inflammatory process, periodontitis stands as the hallmark of our investigation, marked by the progressive degradation of the periodontium and resulting in clinical manifestations such as heightened probing pocket depth, gingival bleeding, and radiographic bone loss. Gingivitis, often acknowledged as a precursor to periodontitis, manifests distinct clinical features linked to dental biofilm, hormonal fluctuations, medications, systemic diseases, and nutritional factors. Our study substantiates these clinical distinctions observed among the healthy, gingivitis, and periodontitis groups, underscoring the significance of comprehending the continuum inherent in periodontal diseases.
The clinical indices such as PI, GI, PPD, and CAL, commonly used in the literature, providing a means of comparison, have also been employed in our study. In a study designed to understand the role of galectin-3, TNF-a, and peptidyl arginine deaminase in periodontal disease, three groups were identified, namely healthy periodontium, gingivitis, and periodontitis. When clinical parameters such as PI, GI, PPD, and BOP were examined, statistically significant differences were found among the three groups [16]. Similarly, in our study, significant differences were observed among all groups in PI, GI, PPD, and BOP data. The oral health data from our study align with the findings of other studies.
Interleukin 1β, a potent pro-inflammatory cytokine, is frequently studied and associated with periodontal disease. In many studies, elevated levels of IL-1β have been found in pockets affected by periodontitis.
There is a clear association between IL-1β and periodontal diseases. A study examining samples from patients with generalized aggressive periodontitis found higher concentrations of IL-1β in GCF compared to serum, with a positive correlation with pocket depth, highlighting the importance of local release in periodontal tissues [17]. In a study by Engebretson et al., where they included 29 non-smoking individuals with different severities of periodontal disease, they planned to measure IL-1β levels in GCF. According to the study’s results, patients with severe periodontal disease showed higher IL-1β levels in shallow pockets of GCF [8]. Stashenko et al. designed a study to determine IL-1β levels in tissues obtained from 12 individuals with destructive adult periodontitis, categorizing tissues into 1) diseased, active, 2) diseased, inactive, and 3) healthy regions. The study concluded that actively diseased areas had higher levels of IL-1β compared to inactive and healthy regions [9]. In another study involving a total of 35 children with periodontal health and gingivitis, the GCF IL-1β levels were evaluated. The study found that the GCF IL-1β levels were higher in the gingivitis group compared to the healthy group [18].
In our study, IL-1β was investigated as a pro-inflammatory cytokine. When compared to the healthy control group, IL-1β levels were significantly higher in the periodontitis groups. The elevated levels of IL-1β align with the literature, supporting that this outcome was an expected result.
Nesfatin-1, a recently discovered adipokine, plays a role in various functions of the body, such as nutrition, appetite, insulin and glucose metabolism, energy balance, regulation of blood pressure, lipid metabolism, coagulation, vascular remodeling, and inflammation [19]. In a study aimed at understanding the protective role of nesfatin-1 in diabetic retinopathy, it was found that nesfatin-1 inhibits the activation of NF-κB/NLRP3 inflammatory signaling and exhibits a protective effect against high glucose-induced inflammation, oxidative stress, and apoptosis in human retinal epithelial cells [20]. Numerous studies have demonstrated the role of Nucb2 and its derivative nesfatin-1 in carcinogenesis. Interestingly, the role of nesfatin-1 in tumor formation appears to be dual, acting both as a metastatic and an anti-metastatic [21]. It has been concluded that nesfatin-1 significantly suppresses NF-κB gene expression, reduces concentrations of TNF-α, IL-1β, and IL-6, and suppresses caspase-3 activity, reducing the number of apoptotic neuronal cells in traumatic rat brain tissues. This finding indicates that nesfatin-1 prevents inflammation [22].
In our study, nesfatin-1 was investigated as an anti- inflammatory cytokine. When compared to the healthy control group, the IL-1β level was found to be significantly lower in the periodontitis groups. The finding of a decreased nesfatin-1 level is in line with the literature, suggesting an expected result. The results of the current study are consistent with other studies regarding the anti-inflammatory property of nesfatin.
Despite the valuable insights gained from this research, it is essential to acknowledge certain limitations that may impact the interpretation and generalization of the findings. Firstly, the cross-sectional design of the study restricts the establishment of causal relationships between the observed variables. Longitudinal studies would be beneficial to discern the temporal associations and potential causal links. Secondly, the study’s sample size, although carefully determined, may limit the generalizability of the results to broader populations. Future research with larger and more diverse cohorts could provide a more comprehensive understanding of the relationships investigated. Thirdly, the reliance on self-reported oral hygiene habits introduces a potential source of bias, as participants may provide socially desirable responses. Utilizing objective measures or combining self-reports with clinical assessments could enhance the accuracy of data regarding oral hygiene practices. Moreover, the study focused on a specific age range (20-60 years), which may not fully represent the entire spectrum of periodontal diseases across different age groups. Including a more extensive age range in future investigations would allow for a more nuanced exploration of age-related variations.
Additionally, the study concentrated on nesfatin-1 and IL-1β, overlooking other potential inflammatory markers that could contribute to a more comprehensive understanding of the inflammatory processes in periodontal diseases. Including a broader array of biomarkers in future studies might provide a more holistic perspective. Furthermore, the potential influence of confounding variables, such as genetic factors, socio-economic status, and lifestyle factors, was not extensively explored in this study. Future research could benefit from a more in-depth examination of these variables to better elucidate their impact on periodontal health.
Conclusions
In conclusion, while this study offers valuable insights into the potential roles of IL-1β and nesfatin-1 in periodontal diseases, the aforementioned limitations highlight the need for cautious interpretation and encourage further research to address these constraints and expand our understanding of the complex interplay between inflammatory markers and periodontal health.


